
For further information: GO2 Foundation for Lung Cancer, Amy Moore, PhD | LUNGevity Foundation, Upal Basu Roy, PhD, MPH | Lung Cancer Foundation of America, Kim Norris | Lung Cancer Research Foundation, Cristina Chin, LMSW, MPH | LungCAN, Kimberly Lester
January 11, 2021 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups
Happy New Year! We recognize that this year is starting on a somber note. We are still witnessing the unfolding holiday surge. As of January 7, we stand at 21.3 million COVID-19 cases in the US, with 361,312 deaths. Some health systems are on the brink of collapse. In LA, over 1,000 people died in less than a week and oxygen is being rationed.
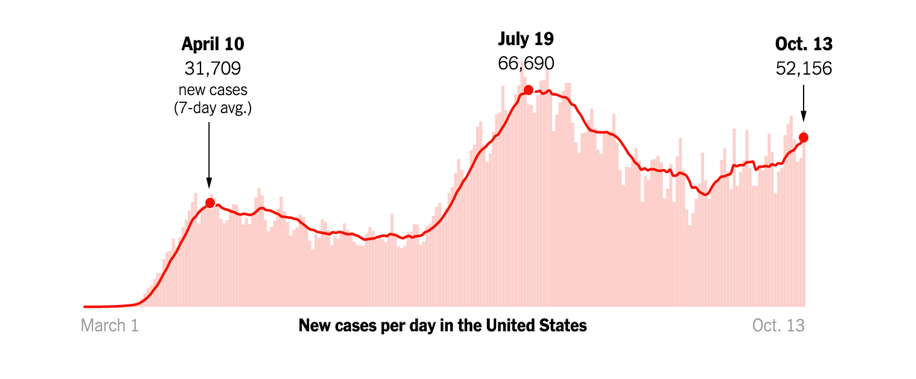
As of our last update on December 14, the Pfizer/BioNTech vaccine had just been granted Emergency Use Authorization (EUA) by the FDA. On December 18, the FDA also granted an EUA to Moderna’s mRNA-based vaccine. As of January 6, approximately 5.3 million people in the US have received at least one vaccine dose, far short of the goal federal officials had set. The below figure shows the percentage of people who have been vaccinated in each state, with light green representing fewer and dark green representing more:
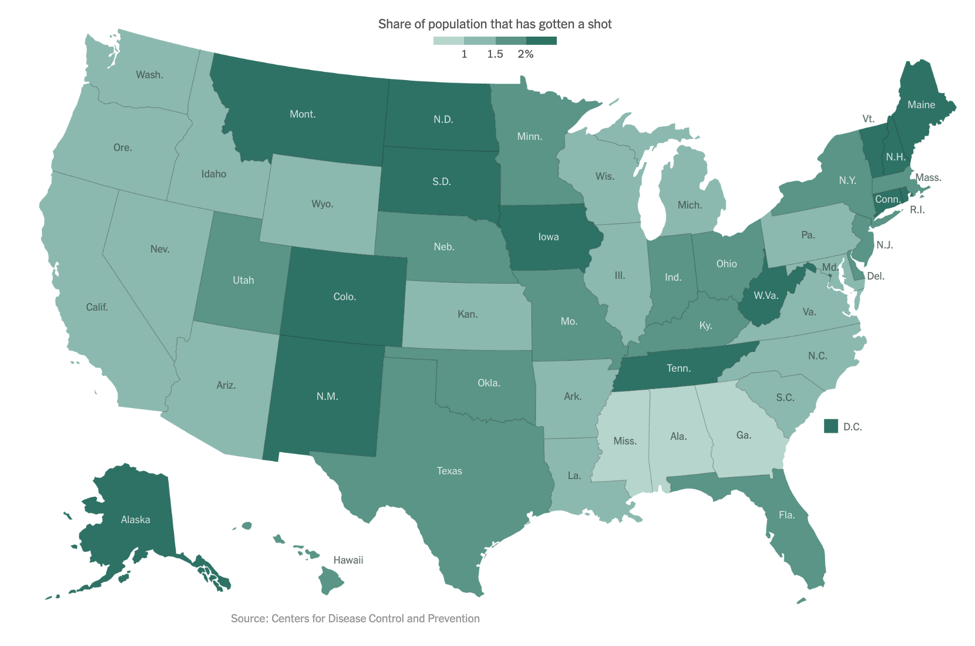
Now that the vaccines are here, many people wonder when they will be eligible to receive one or even if they should get one. By now, you are probably familiar with the concept of the “phased approach” for vaccine roll-out. Initially, there was an effort to vaccinate healthcare workers and other essential workers. Now, there is growing emphasis on vaccinating those over age 75 or with existing co-morbidities.
Prioritization of Patients with Cancer
As patient advocates, we have been actively involved in discussions to urge the prioritization of patients with lung cancer for vaccination against COVID-19. During our hiatus from these updates, the COVID Lung Cancer Consortium, convened by Drs. Fred Hirsch, Paul Bunn and John Minna and representing a global assembly of leaders in thoracic oncology, virology, immunology, vaccines, advocacy, as well as the NCI and FDA, wrote a statement urging officials to prioritize ALL patients with cancer, but especially those with lung cancer for vaccination. All of the leading oncology professional societies have also issued similar statements, including ASCO, AACR, ESMO, and SITC.
Vaccine comparison
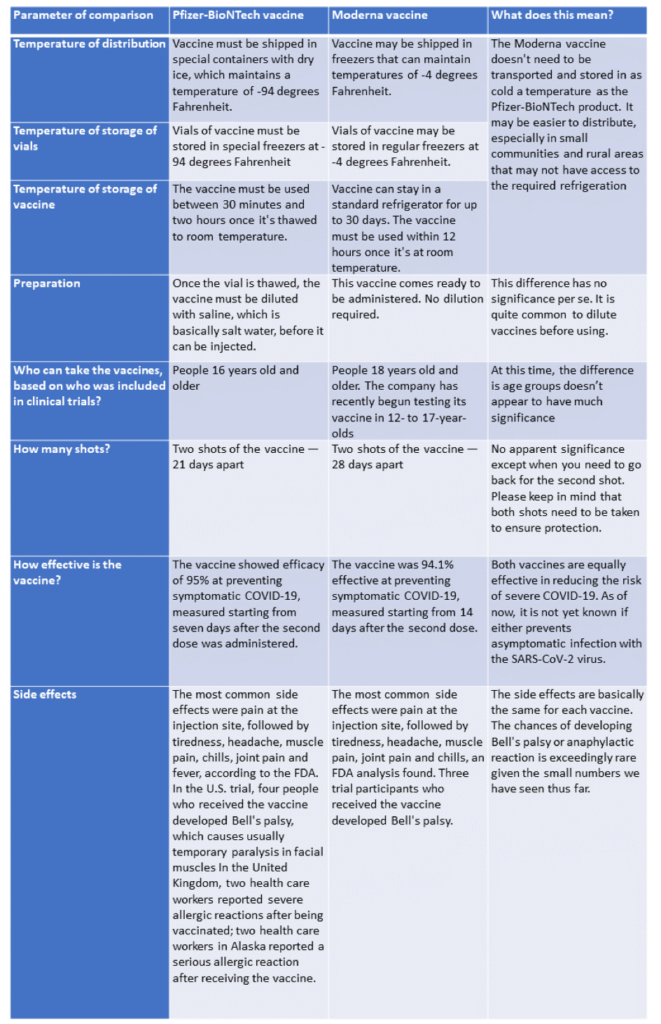
As vaccines become more widely available, people are asking if one vaccine is better than another. Here we offer a quick comparison of the Pfizer-BioNTech and Moderna vaccines (note, we will not discuss the Oxford/AstraZeneca vaccine as it is not yet approved here in the US). As you can see from the table below, both vaccines are equally effective, and the choice of vaccine may be dependent on the state you live in or what your hospital offers.
New COVID-19 variants
There has been a lot of media attention on new variants of the virus that have been detected in the UK, South Africa and even here in the US. Multiple states are now reporting the presence of the variant first detected in the UK. What does all this mean?
The short answer is we don’t fully know. Different viruses with different “constellations” of mutations are arising independently in different locations around the globe. In the case of the UK variant, it appears to be more transmissible i.e. it can pass more easily from an infected person to a non-infected person. There are concerns that the South African variant may not be as susceptible to current vaccines. BUT, we are awaiting more information.
More studies are needed to confirm the impact of these variants on transmissibility (how easily virus spreads between people), infectivity (how easily it enters the body’s cells), disease severity, immune response and vaccine efficacy. It is still expected that current vaccines will offer some degree of protection against the new variants.
What are the immediate implications for our community?
- We should remain vigilant in practicing public health measures, including wearing masks, washing hands and watching our distance.
- If the variants are more easily transmitted, this could overburden our fragile healthcare system, which is under enormous strain from the holiday surge.
- We must increase our efforts around contact tracing and identifying pockets of these new variants.
- We must rapidly increase vaccination efforts nationwide.
December 14, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups
The first case of COVID-19 in the USA was reported on 1/20/2020—over 10 months ago. Since then, the country has reported 15,718,811 cases and 294,535 deaths as of December 12 (per the Centers for Disease Control and Prevention). With 80% of US counties reporting more travel than last year over Thanksgiving weekend in November 2020 despite warnings from the CDC, we are finally seeing the impact of this holiday surge.
- The number of new cases is up more than 20 percent from 2 weeks ago
- The number of hospitalizations has increased by 21 percent
- The number of deaths has jumped 39 percent, with the United States surpassing 3,000 deaths in 1 day for the first time
On December 11, the United States Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the first SARS-CoV-2 mRNA vaccine, BNT162b2 manufactured by the pharmaceutical giant, Pfizer. For a description of how mRNA vaccines work, please check our last update available here. The New York Times reported that large-scale manufacturing and distribution of vaccines has already begun, with the first dosing to start on December 14, 2020. This huge milestone is a positive step towards fighting the COVID-19 pandemic. However, it is important to keep in mind that it will take a considerable amount of time before the entire US population is either vaccinated or immune to COVID-19 through natural infection. With the year-end holidays around the corner and an anticipated increase in travel, the CDC has extended its travel advisory to include the winter break. We encourage our community members to weigh the risks and benefits of travel during this winter. Thanks to the vaccine, the end of the pandemic may be on the horizon. Till such time, maintaining public health measures such as masking, handwashing, social distancing, and minimizing non-essential travel are our best bets for protection.
How was the Pfizer vaccine approved?
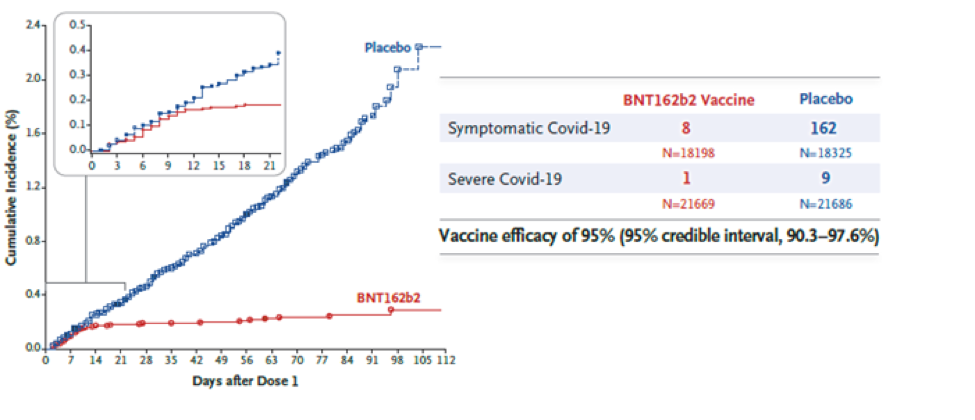
The vaccine was approved based on a randomized, double-blind Phase 2/3 clinical trial. A total of 43,548 participants (older than 16 years) received either two doses of the vaccine or a placebo injection three weeks apart. Participants were followed for safety and for the development of symptomatic COVID-19 for approximately 2 months. Eight participants in the vaccine group developed symptomatic COVID-19, whereas 162 participants in the placebo group developed symptomatic COVID-19. The vaccine was found to be 95% effective in preventing severe COVID-19 symptoms i.e., for every 100 people who received the vaccine, 95 were protected from developing severe COVID-19.
Is the Pfizer vaccine safe?
Side effects reported by trial participants were generally mild or moderate, and reactions were less common and milder in older adults than in younger adults. Those who received the vaccine had localized reactions at the injection site (pain, redness, swelling) and systemic reactions (e.g., fever, headache, muscle ache) at higher rates than placebo recipients, with more reactions following the second dose. Severe fatigue was observed in approximately 4% of vaccine recipients. However, this rate of severe fatigue is also lower than that observed in recipients of approved influenza vaccines for older adults. Serious side effects were similar in both the vaccine and placebo groups (0.6% and 0.5%, respectively).
It is important to keep in mind that we do not have long-term follow-up data from this clinical trial. Sometimes, side effects may show up after months of follow-up. Also, vaccination began in the United Kingdom last week. Two individuals with a history of severe allergic reactions were reported to have had a severe reaction to the vaccine. These individuals carried an EpiPen and use of the pen was sufficient to counteract the allergic reaction. It is anticipated that these reactions will be very rare given that such safety issues were not seen in the large clinical trial. The public health benefits of distributing this vaccine still far outweigh any perceived risks.
What is not known about the Pfizer vaccine?
- We do not know whether the vaccine will be effective for more than 2 months, because participants have only been followed for 2 months so far. However, additional data continues to be gathered.
- Children (less than 16 years of age), pregnant women, and immunocompromised patients (such as those who have received cell-based therapies or chemotherapy for their cancer) were not included in the study. We do not know if the virus will be safe (in children and pregnant women) or effective (in immunocompromised patients who may not mount an immune response) in the groups excluded from the clinical trial.
- The vaccine involves two doses given three weeks apart. The first dose “primes” the immune system to respond while the second dose “boosts” that response. If someone misses the second dose, we do not know whether the vaccine will still be effective.
- We don’t yet know whether the vaccine will prevent the recipient from getting infected or from spreading COVID-19. Again, we need more data. We’ll need to continue practicing public health measures such as masking and social distancing even after receiving the vaccine, at least in the near term.
When will I receive the vaccine?
The United States is adopting a phased approach to roll out large-scale vaccination. The phased approach prioritizes the most essential and the most vulnerable of our population as the first recipients of the vaccine, given the initial limited supply of vaccines. The following figure shows how the state of Massachusetts will use the phased approach for distributing vaccines. It is anticipated that patients with lung cancer will receive vaccines in Phase 1 or 2.
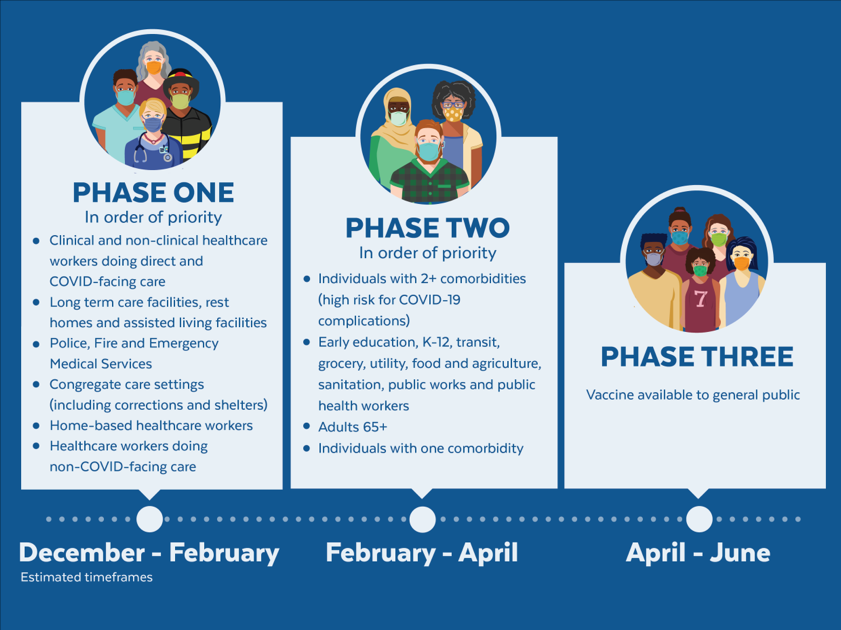
As of December 2020, the Advisory Committee on Immunization Practices (ACIP) recommended that both 1) health care personnel and 2) residents of long-term care facilities be offered COVID-19 vaccine in the initial phase of the vaccination program (Phase 1a).
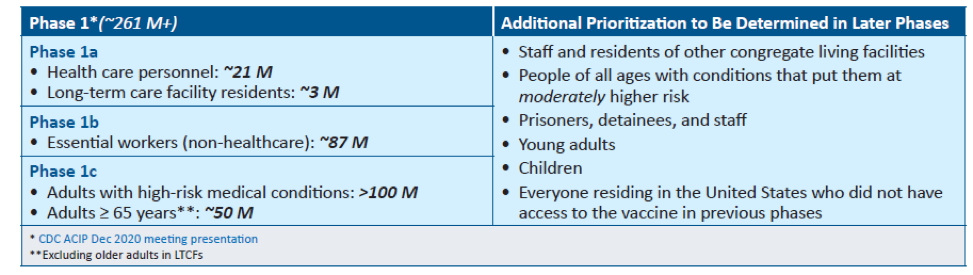
Each state in the United States is likely to have specific vaccination guidelines tailored to their own specific needs. For information specific to your state, please check this link.
An important population for our community is caregivers to patients with lung cancer. If you are the primary caregiver for your loved one, please check your eligibility for receiving the vaccine.
This will be our last update of the year. We wish everyone a safe and peaceful Holiday Season! Please continue to maintain social distancing, wash hands, mask, and minimize non-essential travel. See you in 2021!
November 23, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups
We are at a critical moment in the ongoing COVID-19 pandemic. New cases are rapidly escalating throughout the country, and we are positioned to see explosive growth as people travel and gather to celebrate the Thanksgiving holiday with loved ones. While our understanding of how to treat COVID-19 has grown significantly since the disease first burst onto the scene, deaths continue to mount, with the US now seeing the most daily deaths since May.
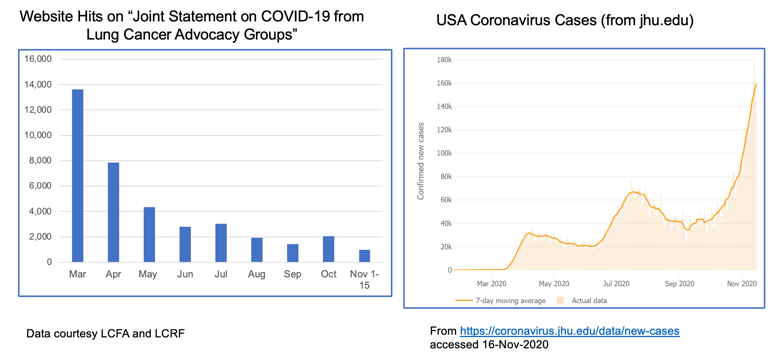
The realities of the current situation are compounded by our collective national fatigue and desire to return to some sense of normalcy. When we look at website hits for these joint statements over time, we see a lot of activity in the spring when COVID-19 was “new,” but those numbers have dropped off substantially through the summer and fall. This stands in stark contrast to the growth of cases through subsequent waves of infection.
The take home message is that we must not let our guard down! Please continue to wear a mask, watch your distance and wash your hands. Our collective actions over the next few weeks CAN make a difference in helping curb the recent surge. We also recognize the importance of balance, particularly for patients with cancer who fear they may not have another Thanksgiving or Christmas. For practical guidance on how to navigate your holidays safely, please refer to this helpful discussion.
Despite the current situation, there is reason for hope. We can now see the light at the end of the tunnel with the recent announcements that both Moderna and Pfizer/BioNTech have developed highly effective COVID-19 vaccines, with others in the pipeline. You can find a comprehensive overview of how vaccine trials work and current vaccine efforts underway here.
Additionally, monoclonal antibody therapies continue to make progress. Eli Lilly recently received Emergency Use Authorization from the FDA for its antibody therapy in recently diagnosed, high-risk patients. Regeneron also received a lot of press when its antibody therapy was used to treat President Trump.
Vaccine FAQs
The development of a new class of mRNA-based vaccines has raised many questions, particularly among the lung cancer community. We have been collecting these questions and will do our best to address them here.
1. How do mRNA vaccines work?
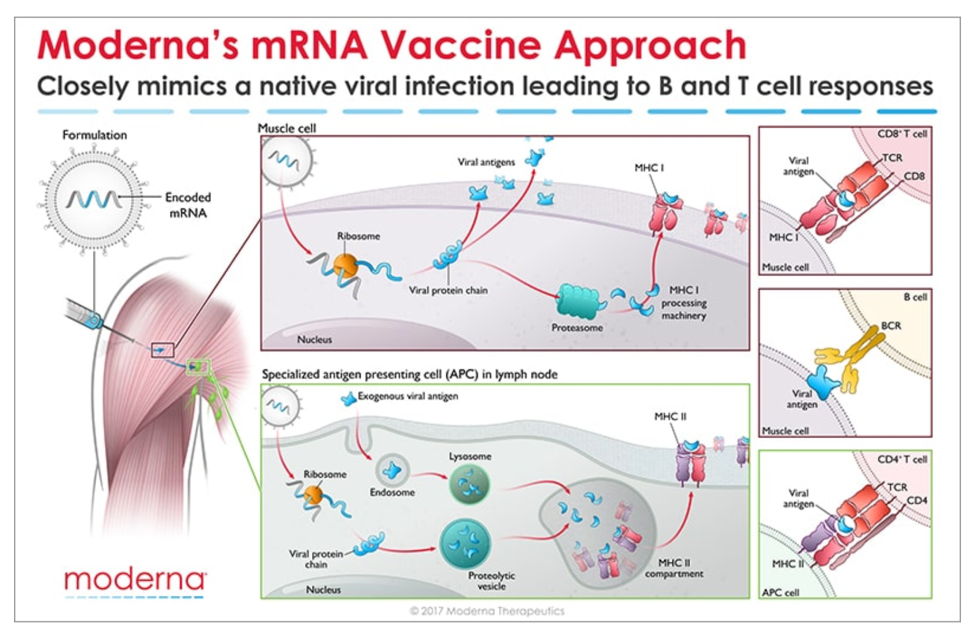
Messenger RNA (mRNA) is the recipe for making a protein. The mRNA gets injected into the body and is taken up by cells that “read the recipe” for making the SARS-CoV-2 spike protein. This is the protein normally expressed as a “crown” on the virus particle and is the part of the virus that binds to the receptor found on cells in the lungs and in other tissues throughout the body. Once these cells take up the mRNA and make the spike protein, they can display pieces of spike on their cell surface to signal the immune cells to become activated. B cells are a type of immune cells that make antibodies that can block virus binding. CD4 T cells support B cells to make antibodies while CD8 T cells can kill virus-infected cells. This is illustrated in the figure below for Moderna’s vaccine (though Pfizer/BioNTech’s vaccine works in the same manner).
2. How do we know these vaccines are safe?
All new drugs and vaccines go through extensive testing as part of the clinical trials process. (summarized in the NYTimes link above). Both the Moderna and Pfizer/BioNTech vaccines are currently in Phase 3 clinical trials, reporting nearly 95% efficacy and no significant safety issues. It is important to note that these trials have been conducted in thousands of patients. However, no significant safety issues does not mean the vaccines don’t come with some unpleasant side effects which are short-lived. Those effects should not be a reason to avoid the vaccine. Educating healthcare providers on the mRNA technology and ensuring them that the vaccines are safe will be key to a successful rollout.
3. When will the vaccines be available? Will patients with lung cancer be prioritized?
Based on the safety and efficacy profiles of both vaccines, it is expected that people will start receiving them before the end of the year, perhaps as soon as December 12 in the US. Many national experts are developing guidance for vaccine distribution, with the National Academies issuing a framework that would see healthcare workers, frontline workers and those in high-risk categories being eligible to be vaccinated first. Given that several studies have now reported high mortality rates in patients with lung cancer who contract COVID-19, it is widely expected that lung cancer patients would be among those first eligible to receive the vaccine in the early stages of rollout.
4. Should I take the first vaccine available or wait for a later generation one?
As stated earlier, both the Moderna and Pfizer/BioNTech vaccines are highly effective with a strong safety profile. There have been fears among many that the rush to produce a vaccine would result in compromised safety or efficacy but adherence to standards established by the FDA and other agencies assures us that these vaccines are safe.
It is important to note that before mRNA vaccines were developed in the fight against COVID-19, they were being developed to help combat cancer. Both Moderna and BioNTech (the company that partnered with Pfizer on its COVID-19 vaccine) have been developing mRNA vaccine technology for some time in the hopes of using this approach to treat various forms of cancer as well as other infectious diseases.
Given the unique threat that COVID-19 presents to the lung cancer community, we strongly encourage you to have a discussion with your doctor about getting the vaccine as soon as it is available to you. As for choosing between these two specific vaccines, the technology is essentially identical. Both require two shots over the course of a few weeks. The differences come down to logistical challenges of ensuring facilities have proper freezers for maintaining the vaccines at the appropriate subzero temperatures.
Other vaccine candidates are in development that use different technology platforms. It remains possible that some future vaccines may require only a single dose (such as Janssen’s vaccine) or be administered differently (intranasal vs injection).
Until those vaccines gain approval, the current decision will be based on availability of the two mRNA-based vaccines.
It is worth noting that a multi-institutional, NCI-funded grant has been awarded to study antibody responses to SARS-CoV-2 infection in lung cancer patients as compared to healthy people. This effort will try to answer why lung cancer patients seem to have worse outcomes from COVID-19 and will study responses in patients receiving a vaccine compared to those who do not.
Unanswered Questions
Several questions remain about the new mRNA vaccines:
- Can these vaccines completely prevent infection, or will they just prevent symptoms from developing?
- Can people who receive the vaccine still transmit the virus to others?
- How long will any resulting immunity last? Previous results from these types of vaccines in other settings suggest that protection may wane after a year.
More data is needed before we can answer these questions.
Final Takeaway
There is no escaping the seriousness of our current national crisis – COVID-19 cases are increasing everywhere and so we must do what we can to protect ourselves and our loved ones a little while longer.
However, hope is on the horizon. We can face 2021 knowing that, through the power of science, this pandemic will eventually come to an end.
HAPPY HOLIDAYS AND PLEASE STAY SAFE!
November 9, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups
The first case of COVID-19 in the USA was reported on 1/20/2020—over 9 months ago. Since then, the country has reported 9,860,558 cases and 237,113 deaths (per Johns Hopkins). As the weather becomes cooler and we spend more time indoors, the number of cases is rapidly accelerating in almost every state. (The virus is holding steady or declining in a handful of states including NY, CA, VT, and ME.)
Given this surge, holiday gatherings and activities present a serious risk for virus transmission. On November 5, 2020, the #LCSM (Lung Cancer Social Media) Chat community on Twitter discussed ways to enjoy and celebrate the holidays safely during the pandemic. Chat participants included lung cancer patients, caregivers, advocates, physicians, and healthcare workers. The chat, which included links to many helpful resources, covered the following topics:
- What have we learned over the past 8 months about how COVID-19 is transmitted?
- How can people reduce the risk of COVID-19 during outdoor activities?
- How can people reduce the risk of COVID-19 when travel is involved? What about travel to or from hot spots?
- How safe is it to meet with family and friends who had COVID-19 and recovered?
- How can people reduce the risk of COVID-19 for indoor activities (shopping, dining in restaurants, family gatherings, worship services, etc.)?
Read the summary of November 5 #LCSM Chat “Celebrating Safely During COVID-19 Pandemic” at wakelet.com/wake/clw31H18j2lOwCQY2MulU.
We hope you find these resources useful as you plan happy and healthy holiday celebrations!
October 19, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups
The daily news reports are a stark reminder that the COVID-19 pandemic is far from over. Consistent with experts’ fears for the fall, new cases are on the rise across the US and in Europe.
Caught in the grips of this unprecedented public health crisis for almost all of 2020, Americans are growing fatigued and restless. The lockdowns in the spring and the extended period of social distancing needed to keep the virus at bay are negatively impacting people’s mental health. For many, it is the lack of touch, a simple hug, that we miss the most.
And this is the time of year when we start looking to Thanksgiving to reunite with family and loved ones, a time often celebrated with large gatherings, extended celebrations and warm embraces. But, at a time when cases are once again surging across the country, each of these activities presents a serious risk for virus transmission. This risk comes at even greater cost for the lung cancer community given the increased likelihood of severe disease and heightened mortality for lung cancer patients who contract COVID-19.
Recently, several health experts have weighed in on how best to approach the holiday to ensure maximal safety. Dr. Anthony Fauci, the nation’s leading infectious disease expert, has suggested Americans need to strongly weigh the risk-benefit of having Thanksgiving gatherings. In places or states with a high number of new cases, some experts even advise canceling (or at least postponing) this year’s celebration. You can check each state’s COVID-19 new case activity here.
While we all feel the need to be close to our loved ones at this time of year especially, we want to urge all of you to do your homework and take appropriate precautions to protect yourselves and those around you. You can use a risk calculator to decide the level of risk. To assist with your planning, the CDC also provides a list of Thanksgiving activities at different risk levels. The table below offers example activities at different risk levels for virus spread.
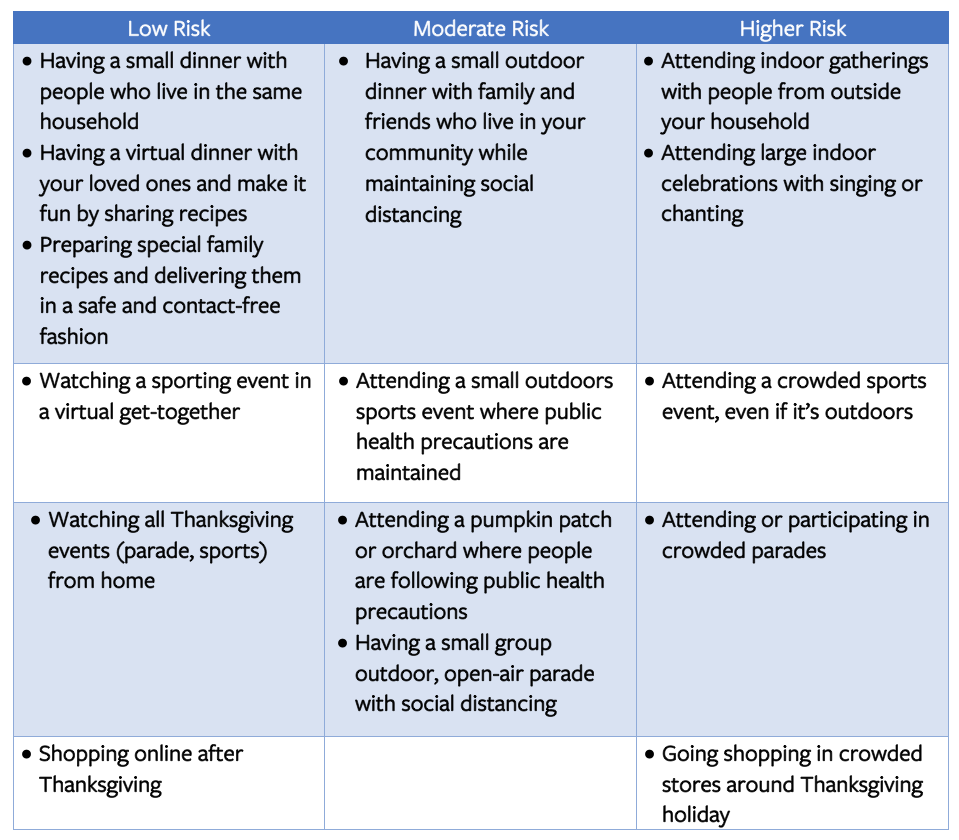
We realize that celebrating the holidays is an important part of our tradition. We, therefore, suggest that you identify an inner circle of family and friends (your social distancing crew) who will be taking precautions with you during the holidays so you can celebrate safely! The holidays can be stressful, and with the pandemic adding a new layer of stress, do not forget to take care of your mental health.
How can you vote safely during the pandemic?
Election day is coming, and it’s important to make your voice heard. If you’re concerned about how to vote safely during a pandemic, Consumer Reports offers a Guide to Voting During a Pandemic that covers several different approaches to voting. The CDC has also issued special COVID-19 safety recommendations for voters. Many of their suggestions are familiar by now; however, the CDC also discusses additional precautions specifically for in-person voting. Some examples:
- Avoid delays by verifying your voter info and having any necessary registration forms ready.
- Bring your own black pen (or stylus, if used in your precinct).
- Review a sample ballot in advance so you can vote and depart quickly.
- Use early voting, if available in your jurisdiction.
- Vote at off-peak times, such as mid-morning.
- If driving to the polls and your schedule allows, monitor the voter line from your car and join it when it’s shorter.
October 5, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups
As of October 3, 2020, the US has had close to 7.2 million cases of COVID-19, with over 200,000 deaths. Daily reported cases of COVID-19 have been on the rise. This is not surprising due to social distancing fatigue and mask fatigue.
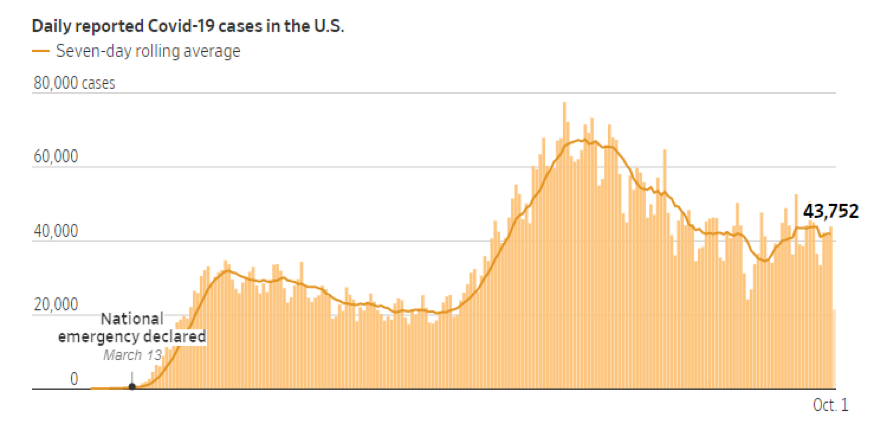
As the weather becomes cooler and we spend more time indoors, an upward trend in COVID-19 cases is expected. Though a lot of vaccine candidates are showing promise in clinical trials, an effective anti-SARS-CoV-2 vaccine will probably not be available for large-scale community use before the middle of 2021. Even once a vaccine becomes available, we will need close to 660 million doses over the next year or so, because the vaccine candidates furthest along in trials require two doses per person. For the near and somewhat distant future, we will continue to rely on public health measures such as washing our hands, maintaining social distancing, and wearing a mask.
As the leaves turn, the holidays begin. Different holidays present different risks – Halloween typically involves large gatherings of children and young people going to door-to-door to collect candy or to party, other holidays bring loved ones together to share meals or celebrate the end of one year and the start of a new one.
Living during the pandemic does not mean we need to completely cancel our holiday celebrations. With advanced planning and maintenance of public health precautions, we can take measures to ensure a safe and COVID-19-free holiday season.
Here are some ideas for celebrating Halloween safely. Additionally, the Centers for Disease Control and Prevention have provided guidelines to ensure that we have a “COVID-free” season.
- Keep a track of community levels of COVID-19 in your area. You can find this information through your local department of health.
- If the weather permits, try to have an outdoor gathering where ventilation is not an issue. If you are planning on having an indoor celebration, it might be a good idea to keep a door or windows open – to promote air circulation.
- Keep the gathering as short as possible. Longer gatherings equal longer time for exposure.
- Smaller gatherings are of course less risky than larger gatherings. Though the CDC doesn’t have specific numbers to guide size of gatherings, they recommend that the size of the gathering be determined by ability to reduce or limit contact between guests (the event space), the risk of spread between guests, and state, local, territorial, or tribal health and safety laws, rules, and regulations.
- If your guests are attending from another state, check the COVID-19 caseload in that state. The same applies if you are planning to travel. It is always a good idea to check caseload at point of origin and destination. If you plan to drive to a holiday gathering and are able to, quarantine for 14 days before travel.
- If you are the host, remind your guests that social distancing, hand washing, and wearing a mask are a part of the celebration.
- The National Institutes of Health has developed a rapid COVID-19 antigen test. If you are able to access a rapid antigen test, it may be a good idea to get tested before you attend a celebration (though since these tests are less sensitive than the nasal swab PCR test and a negative test doesn’t rule out an asymptomatic or presymptomatic infection).
- Since patients with lung cancer are considered at high risk of developing complications from COVID-19, use your judgement and exercise caution when deciding whether you wish to attend a celebration – especially where you do not know a lot of the guests.
We wish everyone a safe and healthy Fall!
September 21, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups | September 21
As of September 18, 2020, the US has had 6.7 million cases of COVID-19, with just over 198,000 deaths. The Midwest is leading new cases, with 8 cities in Wisconsin appearing on The New York Times list of the 20 metro areas with fastest-growing cases.
With the run-up to the US Presidential election now less than two months away, recent weeks have seen a growing national dialog on the potential availability of a SARS-CoV-2 vaccine. In this week’s update, we want to review some basic concepts on vaccines, the clinical trials process for ensuring vaccine safety and provide an update on the current status of the various vaccine candidates currently under development.
What is a vaccine? How long do vaccines last?
In the most basic terms, a vaccine is a substance that can stimulate the body’s immune response to provide protection against diseases caused by different viruses and bacteria. Some vaccines provide potentially life-long protection (measles) while others provide long-term protection but still require periodic “booster” shots (tetanus being a classic example). Still others require annual vaccination because of the nature of the virus – influenza virus (that causes “flu”) undergoes changes from year to year and so the formulation for the vaccine changes each year to accommodate these changes and offer the best protection possible.
(PSA: don’t forget to get your flu shot this year!)
How are vaccines tested?
Everyone feels a great sense of urgency to develop a vaccine for SARS-CoV-2 so we can think about returning to some degree of “normalcy” in our daily lives. A concerted global effort is currently underway not only to develop a safe and effective vaccine but to develop other treatments as well (including so called monoclonal antibodies as well as novel antiviral treatments). In the US, the administration has developed what it refers to as “Operation Warp Speed” to try to accelerate vaccine development.
Without getting into a political debate, we want to offer a brief overview of what goes into getting a vaccine approved. Specifically, once a candidate vaccine is identified, its safety and efficacy (how well it works) must be validated through a rigorous clinical trials process as shown in the schematic below:

For a great overview of how vaccines are developed, the different types of vaccines, how they are tested and the status of current efforts to develop a SARS-CoV-2 vaccine, we refer you to an excellent resource put together by The New York Times.
Vaccine safety
Historically, the United States Food and Drug Administration’s Center for Biologics Evaluation and Research (CBER) has been responsible for regulating vaccines in the US. Recently, the scientific integrity of both the FDA and the Centers for Disease Control and Prevention (CDC) have come into question over fears that they may be rushing vaccine development in the interest of political expediency. Because of this concern, many of the pharmaceutical companies at the forefront of the effort to develop a SARS-CoV-2 vaccine signed an unprecedented pledge affirming their commitment to vaccine safety.
Politics aside, the scientific community must ensure any potential vaccine is both safe AND effective before it is approved and administered to the public. Past experience with the development of SARS and MERS (Middle-Eastern Respiratory Syndrome) vaccines has taught us that coronavirus vaccines need thorough testing. A recent incident that occurred during the Phase 3 clinical trial of AstraZeneca’s vaccine candidate highlights why vaccine safety is paramount. The initial lack of details about the nature of the incident raised concerns about lack of transparency by the drug companies developing these vaccines. In response to mounting pressure, several of the leading contenders have made their protocols public.
Hope on the Horizon
Despite the challenges associated with developing an effective vaccine against SARS-CoV-2, there are several reasons to be hopeful:
- The science is advancing at a historic and unprecedented pace. Previously, the fastest vaccine ever made (against mumps) took four years to develop.
- We have access to novel vaccine development platforms and also experience with coronavirus vaccine development with SARS and MERS. Scientists are building on this pool of available knowledge to develop a vaccine against SARS-CoV-2.
- We have gone from first identifying a novel virus (SARS-CoV-2) as the cause of COVID-19 (Dec 2019) to having the sequence of the viral genome (Jan 2020) and the pursuit of multiple, compelling vaccine efforts within the span of only six months.
September 8, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups | September 8
We hope that all of you had a peaceful Labor Day holiday. This week marks the six-month anniversary of when the World Health Organization declared COVID-19 a global pandemic (March 11). As of September 7, 2020, cases in the US have surpassed the 6 million mark, with over 186,000 deaths.
Nationally, new cases appear to be on a decline but pockets of high COVID activity remain. The figure below shows which states have the most new daily cases and the relative degree of community spread versus containment of the virus:
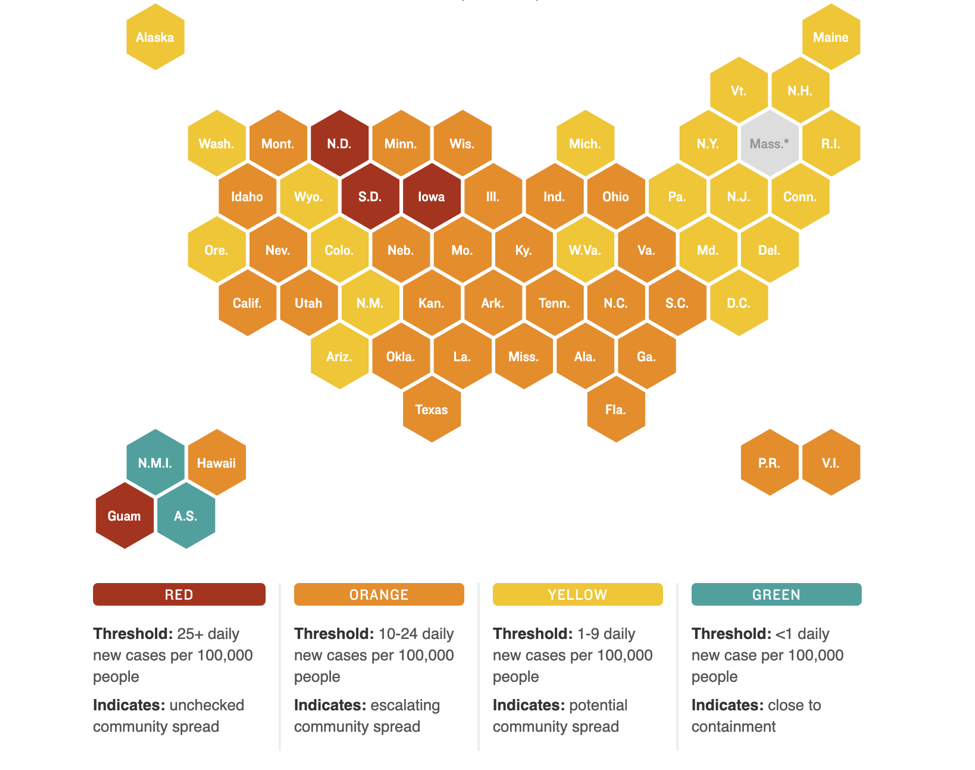
PSA: Get your flu shots!
With the arrival of September, we are strongly recommending that all eligible patients and caregivers get their annual flu shot this year! Public health experts are particularly concerned about the potential for patients to get infected with both influenza and SARS-CoV-2 this winter. Additionally, since the symptoms for these two viruses are similar, many patients experiencing flu-like symptoms may flood already overtaxed healthcare systems. Many doctors’ offices and pharmacies already have flu shots available. It’s also important to remember that it takes approximately two weeks from receiving the shot to have adequate protection. So please make a plan to get your shot as soon as possible.
Some patients, particularly those on checkpoint inhibitors, may be concerned about whether they can take the flu shot – we always recommend asking your doctor but previous studies suggest that it is safe for patients.
We want to hear from you!
We are interested in knowing what topics we should cover in future updates. Please share your thoughts with us by taking this short (1-2 minute) anonymous survey: https://www.surveymonkey.com/r/LungAdvocacy_COVID19_needs
August 24, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups | August 24
It has been more than 6 months since the first cases of COVID-19 hit the United States. We issued our first update on March 3, a week before the World Health Organization declared a global pandemic on March 11. As of August 24, 2020, cases in the United States continue to rise unabated, with over 5.6 million total cases and 175,000 deaths. Countries in Western Europe that had seen a decrease in case load have recently seen small outbreaks, indicating that community spread continues to be a high possibility.
So we are left to wonder: when can we resume normal activities in our lives?
The straightforward answer to that question is when we have achieved a reasonable level of herd (or community) immunity, which occurs when a high percentage of the community is immune to a disease through vaccination and/or prior illness (natural infection). Herd immunity is critical because it not only prevents the spread of infection but also protects people who may not be able to receive a vaccine (for example, the elderly or the severely immunocompromised in whom the immune system is unable to mount a protective response against the virus).
Epidemiologists are hard at work figuring out what levels of herd immunity will protect us from SARS-CoV-2. Initial models suggested that the percentage of people who need to be immune to the virus to achieve herd immunity was around 70%. However, recent research suggests a lower threshold, on the order of only 40%. It is extremely important to keep in mind that no matter the threshold of immunity required, these estimates are based on mathematical models and not true population-based studies.
Our current level of potential immunity to SARS-CoV-2 (the virus that causes COVID-19) is measured using an antibody assay that detects past exposure to the virus whether or not a person had symptoms of COVID-19. Herd immunity through natural infection may depend on location. For example, levels of herd immunity may be lower in rural areas where people are more spread out than in cities, which are more crowded. Also, older people may be more susceptible to the virus and succumb to the disease, whereas younger people may recover from infections and add to the “pool” of herd immunity. Recent research from a COVID-19 hotspot, New York City, looking at the percentage of people who are “antibody-positive” shows a huge variation within the five boroughs of the city. It is therefore possible that the harder hit areas, such as parts of Brooklyn and Queens, may be close to achieving a herd immunity threshold whereas other parts of the city may not (assuming that the antibody tests are accurate and antibodies are long-lasting). This is especially important to keep in mind because it clearly demonstrates that achieving a high percentage of immune individuals through natural infection is not an easy task and comes with a price (please refer to our past update on seropositivity from July 13, 2020).
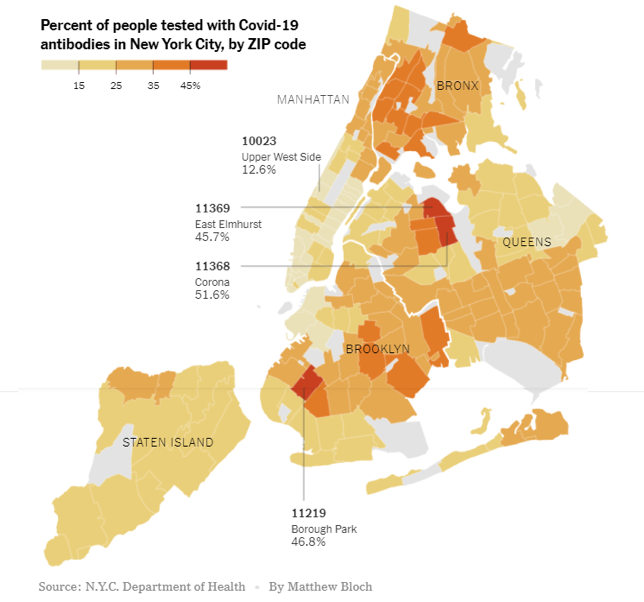
We are interested in knowing what topics we should cover in future updates. Please share your thoughts with us by taking this short (1-2 minute) anonymous survey:
https://www.surveymonkey.com/r/LungAdvocacy_COVID19_needs
August 10, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups | August 10
As of August 9, 2020, we are approaching 20 million cases of COVID-19 worldwide, with almost 5 million cases and 160,000 deaths in the US alone. In this week’s update, we want to shift our attention to another looming healthcare crisis resulting from the pandemic, namely a significant decline in new cancer diagnoses. Given the importance of maintaining appointment schedules, we will also present questions that you may want to ask your healthcare provider in advance of visits to the doctor. Finally, we will highlight ongoing advances in lung cancer research, because cancer doesn’t stop and neither do we.
What is the impact of COVID-19 on new cancer diagnoses?
In the early days of the pandemic here in the US, many stakeholders conducted various modeling simulations to look at the short-term and long-term impacts of the pandemic, particularly related to people continuing to get their recommended cancer screenings (mammograms, colonoscopies). These studies highlighted a looming crisis, predicting a rapid decline in the number of new cancer diagnoses. Dr. Ned Sharpless, Director of the National Cancer Institute, highlighted some of this data in a recent presentation at the AACR COVID-19 and Cancer Conference and in an editorial for Science.
This past week, a new study showed an alarming overall drop (46%) in new cancer diagnoses across six different tumor types, including lung cancer, for the period from March 1 to April 18, 2020:
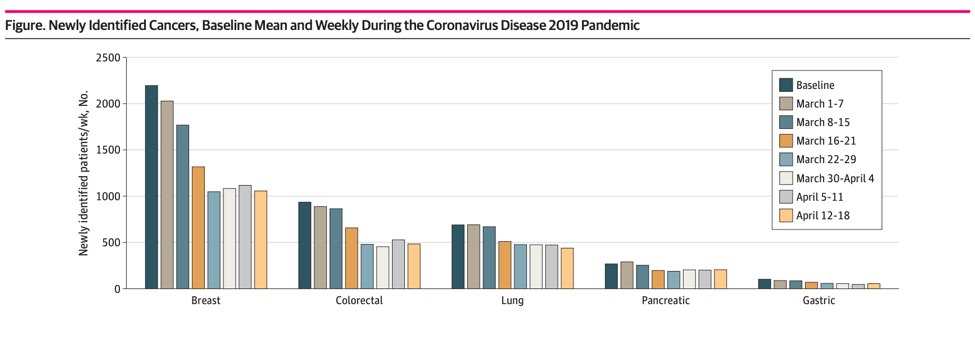
Additional reports from the across the country indicate an even higher drop in new cancer diagnoses. The COVID and Cancer Research Network reported a decline of 74% across 20 sites in the US for April 2020 compared to April 2019.
While people were encouraged to delay these essential screenings during the spring, we know that early detection of cancer is critical for achieving the best outcome and so we want to stress the importance of keeping up with your medical appointments and recommended screenings. To that end, we want to empower you with a set of questions to ask your doctor in advance of any visits so that you feel they are taking appropriate precautions to ensure your safety.
What Should I Ask My Doctor About What They’re Doing to Keep Me Safe?
It’s not unusual to be concerned about the risk of exposure to coronavirus when you go to a clinic or hospital during a pandemic. A facility that is currently experiencing a large volume of COVID-19 patients, or limiting certain procedures or services, may have limitations on which patients it can accommodate. However, most facilities are ready to welcome patients.
Hospital and clinic facilities are taking extra precautions to keep their patients safe. Many facilities are posting videos and information on their websites explaining which precautions they’ve implemented (here is an example video).
If you can’t find information online about the facility you want to visit, call the facility and ask about their precautions. Here are some questions you can ask your care provider or facility before an in-person appointment:
- Can the care provider conduct the visit via telemedicine? (This option requires a patient who doesn’t need an in-person consultation or procedure, AND who is comfortable with and has the equipment for conducting video meetings on a computer or smartphone).
- Can prescriptions be acquired through home delivery, mail order, or curbside pick-up?
- Does the facility require everyone to wear a face covering at all times?
- Does the facility direct patients who have COVID-19 to specific entrances or areas to minimize contact with other patients?
- Does the facility screen all staff for typical COVID-19 symptoms before they start their shifts?
- Does the facility have screeners at patient entrances to ask about known COVID-19 symptoms, take each visitor’s temperature, and ensure appropriate face coverings are worn (and provided, if necessary)?
- Does the facility limit nonessential companions for each patient to no more than a single individual who is free of known COVID-19 symptoms?
- Does the facility promote physical distancing through use of protective barriers, markers on the floor to indicate where to stand to stay 6 feet apart, and separating seats in waiting areas?
- Is each piece of equipment and appointment area cleaned between each use by a patient?
- Do enclosed treatment spaces (like MRI machines) have a waiting period between patients?
- Does the facility adhere to stringent and frequent cleaning protocols, especially in high-touch areas?
- Does the hospital allow visitors in patient rooms? If so, does it require them to check in at a nursing station or other screening area before entering patient’s room?
Additional steps YOU can take to help keep yourself safe before, during, and after a visit inside a hospital or clinic:
- Don a clean face covering before entering the facility, avoid touching it or your face during your time in the facility, and keep it on at all times unless a healthcare provider asks you to remove it.
- Wash your hands frequently. Bring hand sanitizer with you (just in case)
- Before meeting your healthcare provider, wash your hands or use hand sanitizer.
- When you get back to your car or your home, remove the mask carefully by touching only the ear loops. Use hand sanitizer after removing your mask.
- To be extra cautious, wash your hands and face covering and change your clothes when you get home. You might even take a shower. Wash the clothes you wore to the facility.
And lung cancer research continues in full swing!
This year’s World Conference on Lung Cancer (WCLC 2020), hosted by the International Association for the Study of Lung Cancer, went virtual due to the COVID-19 pandemic. Originally scheduled to be held in Singapore from August 8-12, 2020, the scientific sessions will be available from January 28-31, 2021.
WCLC 2020 was officially kicked off on August 8, 2020 with the Presidential Symposium live telecast at 7 PM Singapore time. The Presidential Symposium is a platform to present practice-changing research in the early detection or treatment of lung cancer. This year’s Symposium had three fantastic Phase III trial presentations on immunotherapy for non-small cell lung cancer (NSCLC), a new targeted therapy for ALK-positive lung cancer, and immunotherapy for mesothelioma.
- Currently, a chemotherapy -immunotherapy (pembrolizumab) combination is prescribed as first-line treatment for NSCLC that does not have any targetable driver mutations and that does not express high levels of PD-L1 protein. This is based on the results of the KEYNOTE-189 clinical trial, and the combination is available in the United States and some Western European countries. Results from the Phase III ORIENT-11 trial conducted in China show that addition of an immunotherapy (sintilimab – a PD-1 checkpoint inhibitor) to chemotherapy shows similar benefits seen in KEYNOTE-189. This is an extremely critical finding because results of the ORIENT trial will set the stage for this combination to be available in China and other Asian countries, so that patients can continue to benefit from these advances.
- Ensartinib is a 2nd-generation ALK tyrosine kinase inhibitor. Results from the Phase III eXalt3 trial comparing ensartinib to crizotinib as first-line treatment for ALK-positive lung cancer show that this 2nd generation ALK inhibitor is superior to crizotinib, in terms of its effect both on the primary lung cancer and on brain metastases. These exciting results suggest that ensartinib may be another treatment option for ALK-positive lung cancer in the first-line setting.
- Malignant pleural mesothelioma (MPM) is an aggressive type of cancer affecting the lining of the lungs. It has been associated with exposure to asbestos. Results from the phase III CheckMate 743 trial, comparing combination immunotherapy (nivolumab-ipililumab) to chemotherapy showed that immunotherapy combo is superior to chemotherapy, in the first-line setting.
These three presentations will likely set the foundation for new drug approvals and remind us that lung cancer research will continue, no matter what COVID-19 brings!
July 27, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups | July 27
These updates began on March 3, 2020 — a week before the World Health Organization declared COVID-19 a pandemic — when concerns arose in the lung cancer community regarding news out of China about a novel respiratory virus especially deadly to patients with lung cancer. Dr. Upal Basu Roy (who holds a Masters in Public Health), Dr. Amy Moore (whose PhD research was in virology), and Janet Freeman-Daily (a lung cancer research advocate) led lung cancer patient advocacy groups’ efforts to provide vetted, scientific information with a unified voice. Our goal is to provide a trusted source of information that each member of the community can use to assess their risk and make healthy choices for themselves and their families.
As of July 26, 2020, there have been over 16 million cases of COVID-19 worldwide. This week, the US surpassed 4 million cases– while our nation accounts for just over 4% of the world’s population, we make up 25% of virus cases. Another alarming statistic is the rapid pace with which we keep hitting stark new milestones – it took a mere 15 days for our cases to jump from 3 million to 4 million. These numbers reflect the exponential growth of viruses when appropriate public health measures are not heeded by enough members of the population.
SUMMARY OF AACR COVID-19 AND CANCER CONFERENCE
The American Association of Cancer Research held a special virtual conference titled “COVID-19 and Cancer” on July 20-22, 2020. It is increasingly apparent that cancer and COVID-19 present a unique and unfortunate convergence, with lung cancer patients being among the most at risk for severe symptoms from the disease. This conference grew out of the research community’s need to understand the intersection of these two diseases and reflects the rapid mobilization of cancer scientists to apply their talents to finding solutions to this unprecedented global crisis. As one scientist stated, it is our “moral obligation” to help.
The lung cancer advocacy groups had two “poster” presentations at this conference. The first one summarized the origins of our joint COVID-19 statements and their impact on the lung cancer community. The second one discussed patient concerns that have emerged through these updates and how the advocacy groups can develop programs to address them.
1. What is the latest data on risk of COVID-19 for lung cancer patients?
Several real-world studies were presented at the conference that addressed overall risk for cancer patients as well as lung cancer in particular. Real-world studies rely on data collected from patients receiving treatment at their regular cancer centers or hospitals (i.e. patients not receiving treatment through a clinical trial). Currently, real-world data seems to be the richest source of data for learning about how SARS-CoV-2 (as a virus) and COVID-19 (as a disease) impacts cancer patients.
Registry data is enteredby the patient’s treating physician after the patient has a confirmed diagnosis of COVID-19. Data from two big registries were presented at this conference.
- The CCC19 registry is a multi-institutional, North American effort for all types of cancer. It reported that lung cancer patients were at higher risk of developing a more severe form of COVID-19. Other factors that predicted worse outcomes included older age, poor performance status, presence of co-morbidities, prior or current history of smoking, and a cancer that was progressing. The CCC-19 study showed an overall mortality of 26% for lung cancer patients with COVID-19, the highest of all the cancer types analyzed.
- The TERAVOLT registry is a multi-institutional, international effort dedicated to thoracic (lung-related) cancers. TERAVOLT data on 400 COVID-19 patients showed overall mortality of 35.5% for patients who had lung cancer and a higher mortality of 41% for patients who have SCLC. This increases the challenges presented by the pandemic to rural communities in the Southeast, where SCLC burden is high. Poor performance status was associated with more severe COVID-19 symptoms for SCLC patients. The patients in this study are primarily European, where the standard of cancer care may be different than in the US. It is important to keep in mind that SCLC is highly aggressive and has a higher symptom burden than NSCLC.
Single-institution data provide convenient samples to understand the natural history of a specific disease. At the conference, data from Memorial Sloan Kettering Cancer Center in New York City showed that prior immunotherapy for lung cancer did not impact outcomes of SARS-CoV-2 positive lung cancer patients. This data seems to contradict other registry-based efforts which have suggested that immunotherapy may predict worse outcomes. At the height of the pandemic in NYC, 20% of MSKCC’s lung cancer patients with COVID-19 died but many, including those with late-stage cancer, recovered. This study suggests patient-specific factors (such as type of treatment and patient characteristics) may determine overall risk and susceptibility to worse outcomes. It is important to keep in mind that standard of care and patient characteristics may be unique in a specific institution and therefore the results may not be generalizable.
One study presented at the conference that looked at electronic health records of patients in the US showed that an active cancer diagnosis coupled with co-morbidities such as diabetes and hypertension predicted worse outcomes for COVID-19.
Some common themes emerged for lung cancer patients:
- Patient-specific factors such as older age, presence of lung comorbidities such as COPD, and a poor performance status (higher than 1) are associated with a risk of developing a more severe form of COVID-19.
- Certain treatments such as chemotherapy (either alone or in combination) may increase the risk of developing a severe form of COVID-19 due to the immunosuppressive effects of chemotherapy.
We are still learning about how patient-specific factors and treatment-specific factors related to lung cancer can influence the severity of COVID-19. It is best to discuss how an individual patient’s situation will be impacted with the treating physician.
What is abundantly clear at this point is that multiple studies point to increased risk and worse outcomes in lung cancer patients with COVID-19. As the pandemic continues to spread throughout the US, it is imperative that lung cancer patients continue to take the threat seriously and take appropriate steps to protect themselves and those around them:
- limit unnecessary travel (particularly to areas where COVID-19 is prevalent),
- practice social distancing,
- wash hands frequently (or use hand sanitizers when handwashing is unavailable), and
- WEAR A MASK when out in public.
2. How has the COVID-19 pandemic impacted oncologists and the cancer healthcare community?
The impact of the COVID-19 pandemic on the mental health of oncologists cannot be underestimated. Several studies suggest that oncologists will likely suffer from “burn-out” syndrome and post-traumatic stress disorder (PTSD). Two studies documenting the effect of the pandemic on mental health of oncology professionals were presented at the conference.
- One study looked at 300 oncologists in Western Europe and the United States during the first phase of the pandemic. Two biggest fears reported by the oncologists (almost 75% of participants) were “fear that their patients would get sick” and “fear that their family members would get sick.” Several oncologists opted to live away from their families during their oncology service to protect their families (Symposium 7, Dr. Gabriella Pravettoni).
- In the second study reported at the Keynote Symposium, which included 1570 oncologists from 102 countries, more than 75% of the oncologists reported that they feared contracting COVID-19 (July 21 Keynote, Dr. Solange Peters).
Both these studies highlight the importance of developing adequate mental health support services for healthcare professionals as the effects of the pandemic emerge.
As patients and advocates who work regularly and intimately with oncology healthcare professionals, we must not forget to express our gratitude to all members of the patient care team.
July 13, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups | July 13
As of July 1, 2020, more than 10 million people worldwide have been infected with SARS-CoV-2, the virus that causes COVID-19. In the United States alone, more than 3 million people have tested positive, as of July 10, 2020. Our knowledge about how the virus affects our immune systems and other organs is continuously evolving. Along with this knowledge, doctors are becoming better at managing patients with a confirmed diagnosis of COVID-19. However, it is important to keep in mind that the virus is still infectious.
In this update, we answer some key questions about the current need for public health measures, testing in light of the recent rise in COVID-19 cases, what the test results means, some recent press on “new strains” of SARS-CoV-2, and finally what this means for herd (community) immunity.
What public health measures can help stop the spread of the virus?
Current data still suggest the virus is most commonly spread person-to-person, via droplets expelled by talking, coughing, or sneezing while in close face-to-face contact. The virus may also spread via aerosols (smaller droplets that remain suspended in air) but at this point, this has not been clearly established. People can have an active case of the virus and show no symptoms (asymptomatic spread). Until a vaccine is available, we need to take action to prevent transmission of SARS-CoV-2 through these strategies:
- Personal hygiene (e.g., hand washing)
- Testing people to identify cases of active infection
- Using distance or physical barriers to reduce the spread of infectious droplets (e.g., staying home, social distancing, wearing masks, isolating people who are infected)
- Contact tracing (e.g., notifying people when they have been in contact with someone who has active infection)
- Government-level actions (e.g., governmental limits on sizes of gatherings or business capacity; school or workplace closures; stay-at-home orders)
- Travel restrictions (e.g., border closure, enforced quarantine on visitors from infected areas) if required
Should I get tested for COVID-19? Which test is right for me? What do the test results mean?
If you or your loved one suspects that they have been exposed to SARS-CoV-2, and/or have developed the three most common COVID-19 symptoms (fever, cough, and shortness of breath), we recommend you get tested.
Currently, three tests are available for COVID-19. The choice of test depends on whether you suspect that you have an active (existing) infection, or you were infected in the past and want to confirm infection.
| Stage of infection | Current infection | Past Infection | |
|---|---|---|---|
| Type of Test | PCR test | Antigen test | Antibody test |
| How is a sample collected? | A nasal or throat swab | A nasal or throat swab | A blood sample |
| What does a positive test result mean? | You have an active SARS-CoV-2 infection. Even if you do not have symptoms, a positive result may suggest you can infect others. | You have an active SARS-CoV-2 infection-. Even if you do not have symptoms, a positive result may suggest you can infect others. | You were possibly exposed to SARS-CoV-2 in the past, even if you did not have major symptoms. HOWEVER, this does NOT necessarily mean you have immunity to the virus (we are still learning how long immunity might last). |
| What does a negative test result mean? | You might not be currently infected with SARS-CoV-2. HOWEVER, this does NOT necessarily mean you don’t have a current infection – especially if you display symptoms. Your doctor will take into account the entire clinical picture and not just test results. | You might not be currently infected with SARS-CoV-2. HOWEVER, this does NOT necessarily mean you don’t have a current infection – especially if you display symptoms. Your doctor will take into account the entire clinical picture and not just test results. | You might not have been exposed to SARS-CoV-2. HOWEVER, this does NOT necessarily mean you were not exposed in the past. It is becoming increasingly clear that antibodies against SARS-CoV-2 do not last for a very long time. Therefore, timing of test matters. |
More testing will help us to identify more people who have an active case of COVID-19 and may be able to spread the disease, whether or not they have active symptoms. An accurate count of active cases tells us where the virus is currently spreading and hopefully helps us to implement prevention measures in time to limit spread of the disease in that area.
Has the SAR-CoV-2 virus mutated? Is this new mutation more infectious? What does this mean for prevention, vaccines, and treatment?
A preliminary manuscript (which has not yet undergone peer review) describes the emergence of a new mutation seen in a specific gene of the SARS-CoV-2 virus. This mutation, which was first discovered in Europe, is called D614G. It causes an increase in the number of spike proteins in the virus. Since the spike protein is how the virus attaches to human cells, the authors concluded that this mutation makes the virus more infectious. However, it does not appear to make the resulting disease more severe or deadly. Currently, the real-world implications of this mutation and its impact on the development of vaccines and treatments are still unclear.
Are blood tests detecting coronavirus antibodies more frequently?
Many countries are using blood tests to look for SARS-CoV-2 antibodies in their populations. Testing of blood serum is called serology. The percentage of individuals in a population that have these antibodies in their blood serum is called seroprevalence. As COVID-19 spreads across the globe, different areas will have different levels of seroprevalence.
The CDC is now conducting large-scale geographic seroprevalence surveys at a number of sites across the country. Initial results from the first six sites showed rates of people who tested positive for SARS-CoV-2 antibodies varied from about 1% (in WA state) to about 7% (in greater NYC area).
Several global seroprevalence studies have been published recently. In Spain, which was hit hard by COVID-19 in the spring of 2020, approximately 5% of people in the 36,000 households tested had antibodies against SARS-CoV-2 (they are “seropositive”—their serum tested positive for antibodies). The seropositive rate is closer to 10% near Madrid but only 3% along the coast. Given that 95% of Spaniards do not have antibodies (seronegative), the authors conclude that it is important to maintain the public health measures described above.
A second study from Brazil also found regional variability in seroprevalence, with an overall seropositive rate of 1.4%. However, surprisingly, some cities along the Amazon had some of the highest rates reported so far, approaching 25%. This finding further counters the argument that SARS-CoV-2 is susceptible to heat, since Brazil maintains a hot, tropical climate.
What about herd immunity?
Herd immunity (or community immunity) occurs when a high percentage of the community is immune to a disease through vaccination and/or prior illness. We currently face several challenges to achieving herd immunity. First, seropositivity rates remain significantly below the ~70% required to achieve herd immunity, even in hotspot areas such as NYC. Second, a growing number of reports suggest that antibody levels fall off significantly as early as 8 weeks after infection (though other features of the immune system may provide some protection).
Some have suggested that public health efforts to reduce transmission are only delaying the acquisition of herd immunity. Sweden has been held up as a model for keeping a country open to develop herd immunity. However, Sweden serves more as a cautionary tale – it experienced much higher death rates than its Scandinavian neighbors yet was not spared the economic impact of the pandemic. Various models have suggested that efforts to achieve herd immunity by natural infection (ie, letting the virus run its course without vaccines) would result in over 30 million deaths globally.
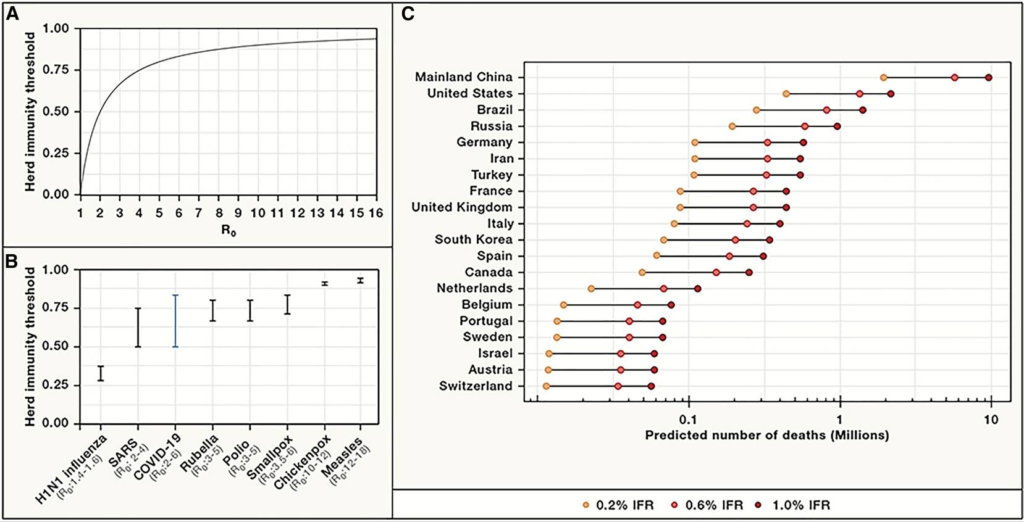
Letting the virus run its course comes at extraordinary cost in terms of human lives. Further, given the low rates of seropositivity among areas hard-hit by the virus and the rapidly declining antibody levels in individuals, it seems unlikely that we will achieve herd immunity WITHOUT a vaccine.
How risky is returning to “normal” activities?
These updates are intended to give you the latest evidence on what we know and to provide a framework for you to make your own decisions as we all learn how to navigate this new “normal.” In that spirit, we share this recent graphic that helps assess the relative risk of various daily activities:

June 27, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups | June 27
As of June 28, 2020, the United States has reported more than 2.5 million cases of COVID-19 and 125,484 deaths. We are now seeing a rapid escalation in cases in states across the US. Some would argue that these increases simply reflect more testing but that only tells part of the story. Perhaps a more meaningful metric is the rate of new hospitalizations and ICU bed capacity. Seven states (AZ, AR, CA, NC, SC, TN, TX) are now reporting their highest hospitalization rates since the pandemic started. In hard-hit Houston, TX, ICU bed occupancy stands at 97% at Texas Medical Center. Though only a quarter of that number is currently due to COVID-19 cases, there is once again growing concern about the ability of our hospitals to handle the rapidly increasing number of patients, especially once a second wave of infections strikes.
There are also some changing demographics with this most recent uptick in cases, including growing number among young adults ages 20-30. While that may seem to be good news at first, since younger people for the most part have a less severe form of the disease than the elderly or those with underlying comorbidities, this also creates a potential reservoir of the virus that could rapidly extend to more vulnerable populations in the surrounding community.
In the absence of a vaccine or an effective treatment, our best modes of protection remain continued social distancing, frequent handwashing, and wearing masks or facial coverings. This paper from The Lancet supports the use of face masks in reducing transmission in both the healthcare and community setting. The lack of a spike in cases related to recent national protests also suggests that masks played a large role in preventing transmission of the virus. As cases continue to rise across the country, more and more states are beginning to mandate the use of masks or facial coverings, as shown below:
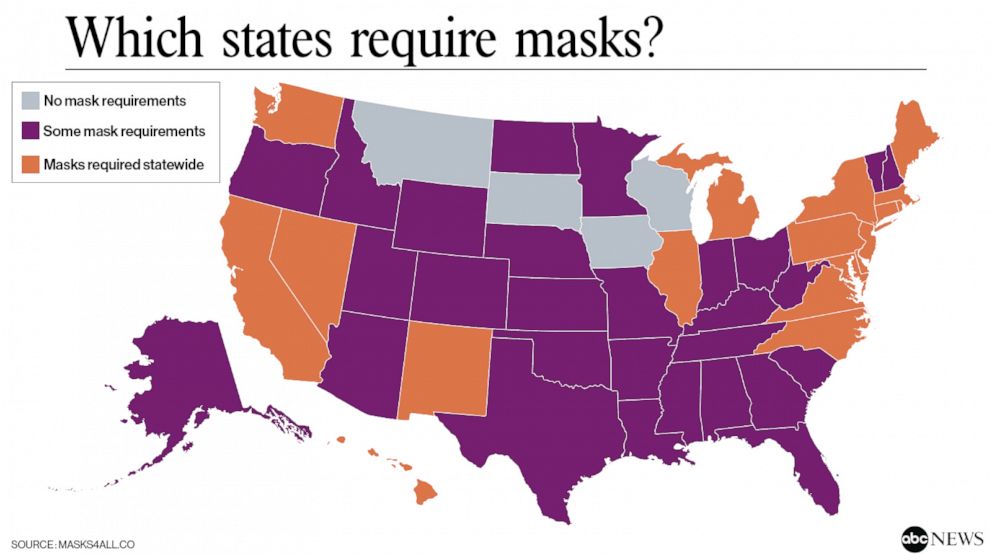
Additional studies on outcomes, antibody response, and radiological findings:
- In our June 15, 2020 update, we presented findings from the TERAVOLT study, which has reported an increased mortality rate (33%) in lung cancer patients with COVID-19. Some have questioned this study’s findings and how translatable they are to the situation here in the US. New data from Memorial Sloan Kettering Cancer Center (MSKCC) in NYC were reported for a cohort of 102 patients with both lung cancer and COVID-19. Of these patients, 62% were hospitalized and 25% died. Of the patients who required ICU level care (21%), 72% died. However, COVID-19 severity appeared to correlate more with patient-specific factors rather than tumor-specific characteristics or treatments. Thus, while this is a small study, it does reinforce the vulnerability of lung cancer patients to COVID-19. Another study from Memorial Sloan Kettering Cancer Center looked at a cohort of 423 cancer patients with COVID-19 (8% of which were lung cancer patients) and found that 20% developed severe respiratory illness (including 9% who required mechanical ventilation) and 12% died within 30 days. In addition, the authors found that administration of immunotherapy was associated with a higher risk of complications. Despite small sample size of patients from single institutions and from different countries, all these studies reinforce two points: cancer patients may be at a higher risk of developing complications from COVID-19 and various patient- (such as lung damage from radiation therapy) and treatment-specific (immunosuppressive treatments such as chemotherapy) factors determine the extent of severity.
- New research out of China suggests that the antibody response (a measure of immunity) to SARS-CoV-2 infection may not last as long as for other respiratory viruses, particularly among asymptomatic patients. The study, published in Nature Medicine, suggests that antibody levels fall off by over by 70% in both asymptomatic and symptomatic patients by 8 weeks following infection. Though the sample size is small, if true, these results have important implications for establishing “herd immunity” (also sometimes referred to as community immunity) through natural infection as well as vaccination efforts.
- Additionally, the paper above described radiological imaging findings in the lungs of asymptomatic patients, including ground-glass opacities as shown below. Coupled with prior reports of extreme lung damage in some patients (including a healthy 20 year old woman who required a double-lung transplant), these data, though from a small cohort of patients, affirm that there is still much we do not know yet about COVID-19’s impacts and if infection has a lasting impact on lung function in patients who recover. In the case of lung cancer, the overlap between radiological findings in COVID-19 and lung cancer complicates diagnosis, treatment and management of patients.
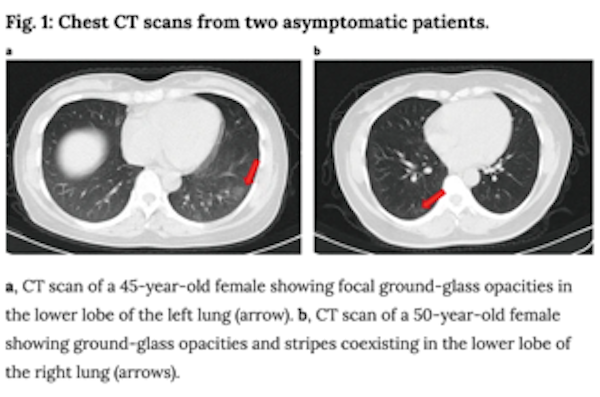
In light of these studies and others which suggest an increased risk for patients with lung cancer, researchers from the fields of lung cancer, virology, immunology and epidemiology are rapidly mobilizing to create large-scale programs to address questions such as:
- What is the relative risk of COVID-19 for lung cancer patients?
- How many lung cancer patients have been infected with SARS-CoV-2 and have antibodies against the virus?
- What are the features of the immune response to SARS-CoV-2?
- What are the long-term implications for lung cancer patients who recover from COVID-19?
In summary, we continue to advise our community to maintain public health precautions as they go about their daily activities such as household chores and groceries. In a recent New York Times article, former director of CDC (under the Obama administration), Dr. Tom Frieden says, “Start with the three Ws: wear a mask, wash your hands, and watch your distance.” Now more than three months into the pandemic, hospitals and clinics have excellent procedures in place to ensure that patients are kept safe during clinic appointments. We strongly advise lung cancer patients to check with their doctors on what these precautions are, in case they are concerned about getting exposed to SARS-CoV-2 while seeking healthcare. It is not advisable to miss clinic appointments without consulting your healthcare team.
AACR Virtual Conferences
Lung cancer patient advocates attended AACR’s Virtual Annual Meeting II on June 22-24. As expected, many presentations focused on the intersection of COVID-19 and cancer as well as our current national dialog on racial issues. Dr. Lisa Newman presented work on the double hit minority cancer patients are facing as a result of the ongoing pandemic. Dr. Ned Sharpless, Director of the National Cancer Institute (NCI), reported data predicting an additional 10K cancer deaths over the next decade as a result of missed screenings, delays in diagnosis and reductions in cancer care. Though these models were for breast and colorectal cancer, there is equal concern about the potential impacts on lung cancer. The lung cancer advocacy groups must continue to push forward policies that protect minority communities and ensure access to continued screening and care during the current crisis.
Thank you to everyone who participated in our recent survey to collect data on the value of these updates and patient concerns that have emerged as a result. We are pleased to report that we have had two abstracts accepted for presentation at the upcoming AACR Virtual Meeting: COVID-19 and Cancer being held July 22-24. Our community will be well-represented as we learn even more about the intersection of these two diseases and the implications for lung cancer in particular.
June 15, 2020 | Download the statement as a PDF
Update to the Joint Statement on COVID-19 from Lung Cancer Advocacy Groups | June 15
As of June 12, 2020, the United States has reported more than 2 million cases of COVID-19 and 113,914 have died from this disease [www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html]. States are in different phases of reopening and shelter-in-place restrictions and lockdown have been eased in almost every state in the USA. With restrictions being lifted despite the upsurge in new cases, a big question remains.
Is it safe to return to routine activities? The short answer to this question is NO – we are not yet ready to return to routine activities.
In this week’s update, we provide evidence on why the lung cancer community needs to be vigilant about the risk of exposure to SARS-CoV-2, the virus that causes COVID-19. We also describe the impact of easing shelter-in-place restrictions in different states in the US and conclude by providing expert guidance from epidemiologists on what to expect over the next year.
- Lung cancer patients are at higher risk of developing complications from COVID-19: The Thoracic cancERs international coVid 19 cOLlaboraTion (TERAVOLT) registry study is specifically tracking outcomes for lung cancer patients infected with COVID-19. Recently published data [www.thelancet.com/journals/lanonc/article/PIIS1470-2045(20)30314-4/fulltext] from this study suggests that stage IV non-small cell lung cancer (NSCLC) patients are at higher risk of complications and mortality if they get infected with SARS-CoV-2. Of the patients included in the study, 33% succumbed to complications from COVID-19. Though the data generated for this study is primarily from European countries, it is highly probable the findings will hold true in other high-income countries such as the United States. Though the study does not provide information on the outcomes of small cell lung cancer (SCLC) patients, we anticipate that the findings will hold true for SCLC as well, given the high symptom burden of SCLC. Also, the TERAVOLT study has identified smoking history as an important predictor of developing complications from COVID-19. This suggests that SCLC patients may be at higher risk of a severe form of COVID-19, given the association of SCLC with active tobacco exposure.
It is important to keep in mind that the CDC considers patients with lung co-morbidities (such as lung cancer) to be at a higher risk of developing complications from COVID-19. - Easing shelter-in-place restrictions has led to an escalation in new COVID-19 cases in the United States: It is now proven that public health measures such as home isolation, business closures, and other large-scale social distancing measures have had large and measurable health benefits in containing the spread of COVID-19 and “flattening the curve”, as described by a recent research study [www.nature.com/articles/s41586-020-2404-8] in the journal Nature. Therefore, before lifting or removing these restrictions, there needs to be careful deliberation taking into account the local case load of COVID-19 and availability of critical hospital resources, should there be a spike in cases when restrictions are lifted. In order to assist states in reopening, the CDC has suggested a phased-approach[https://www.cdc.gov/coronavirus/2019-ncov/downloads/php/CDC-Activities-Initiatives-for-COVID-19-Response.pdf] to easing shelter-in-place restrictions. However, it is becoming increasingly apparent that we will need to monitor reopening with caution and continue to maintain public health precautions.
- The state of Florida reported a spike in COVID-19 cases since the state entered phase 2 reopening on June 5th. The 64 counties that moved into the second phase of reopening saw a near 42% increase in new cases [https://www.miamiherald.com/news/coronavirus/article243349186.html] the week before that could not be explained by increased testing alone.
- The state of Arizona has seen a huge spike in the number of COVID-19 cases since the state eased restrictions at the end of May. Arizona’s Department of Health Services has reported [https://www.newsweek.com/arizona-icu-capacity-reaches-80-percent-state-records-single-day-high-1510594] that the state has already reached 80% of its ICU bed capacity.If you are curious to see how your state is performing in light of the recent lifting of shelter-in-place restrictions, please check out this article [time.com/5852913/covid-second-wave].
- We should continue to maintain public health measures to minimize exposure to SARS-CoV-2: Easing shelter-in-place restrictions does not mean we should stop maintaining public health precautions. We highly recommend that everyone:
- Wear masks in public. A recent publication [www.pnas.org/content/early/2020/06/10/2009637117] in the Proceedings of the National Academy of Sciences shows wearing masks is protective, given that transmission of the virus through air is one of the primary means of infection.
- Continue to maintain six feet distance from others in public
- Continue to practice social distancing
- Self-quarantine in case you suspect you may have been exposed to the virus
- Wash your hands regularly with soap and water
- Avoid touching your face
- Avoid large gatherings of people
- Minimize all non-essential travel
As a lung cancer patient or caregiver, if you have any questions on how to maintain public health measures as you run errands and go to work, please check out the CDC resources here [hwww.cdc.gov/coronavirus/2019-ncov/daily-life-coping/going-out.html] . We are also learning about the long-term effects of an infection. Impact of COVID-19 on the body can last for several months [www.theatlantic.com/health/archive/2020/06/covid-19-coronavirus-longterm-symptoms-months/612679] . In some extreme cases, damage to the lungs is severe enough to require a double-lung transplant [www.nytimes.com/2020/06/11/health/coronavirus-lung-transplant.html]. We therefore firmly believe that it’s better to be safe than sorry!
- Epidemiologists suggest that the timeline for resuming different activities will be determined by the availability of a vaccine against SARS-CoV-2: In a recent article in the New York Times [https://www.nytimes.com/interactive/2020/06/08/upshot/when-epidemiologists-will-do-everyday-things-coronavirus.html], 511 epidemiologists were asked to rate how soon they would resume different activities. Below are the results of this opinion survey. Though this data is not meant to serve as guidelines for the general public, it gives us a picture of where expert opinion lies with regard to when to resume normal activities.

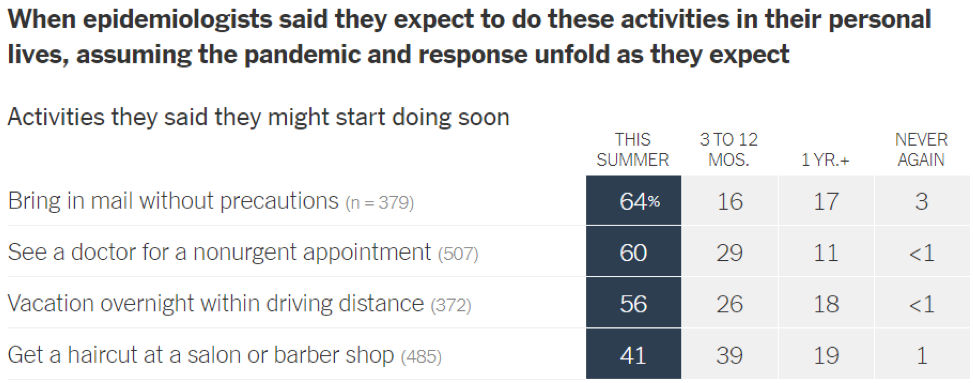
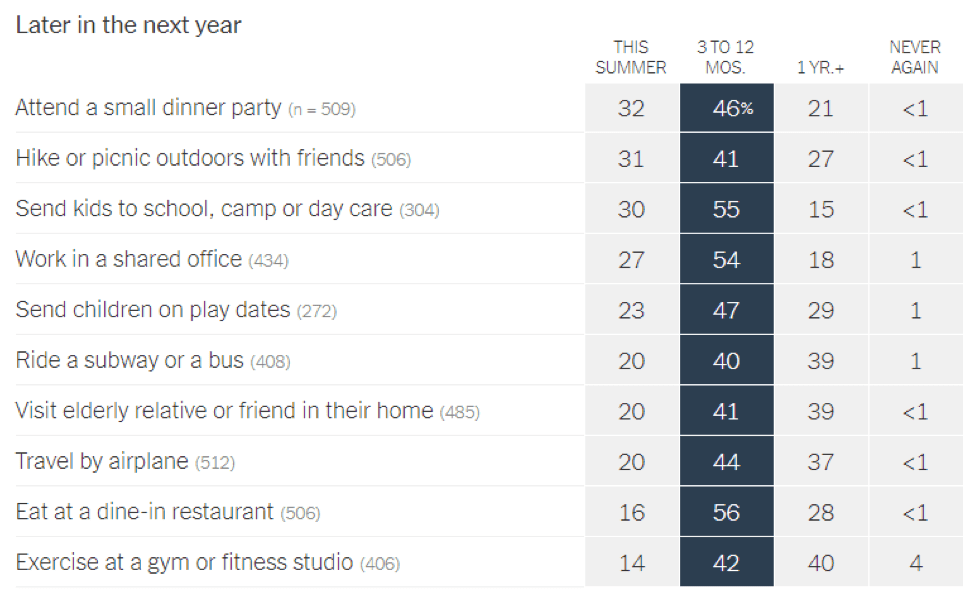
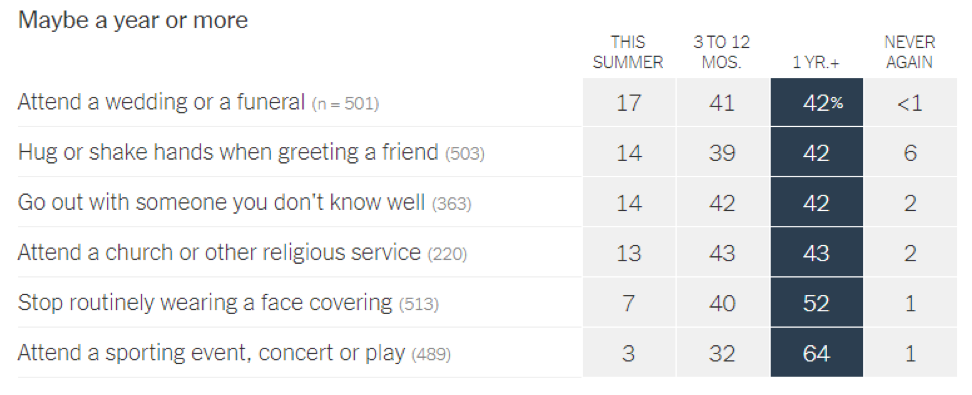
June 1, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | June 1
This past week marked a grim milestone in the United States, as we officially surpassed 100,000 deaths [https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html] from COVID-19. Our groups continue to recommend that the lung cancer community adhere to best practices to limit exposure, including wearing masks/face coverings when out in public, frequent handwashing, ongoing social distancing, and limiting non-essential travel.
Normally at this time, representatives from our respective organizations would be in Chicago for the annual American Society of Clinical Oncology (ASCO) meeting, for which over 40,000 oncology professionals gather to share best practices in clinical oncology research and academic and community practice. In light of the ongoing pandemic, ASCO 2020 was held as a virtual conference.
Note: There are many exciting updates and recent FDA drug approvals in the lung cancer space. These are being shared via other channels through our respective organizations and will not be covered here since our goal is to focus exclusively on relevant COVID-19 updates for the lung cancer community.
In this week’s update, we will cover three topics:
- COVID-19 presentations from ASCO 2020
- Advocacy groups participate in IASLC “Lung Cancer Considered” podcast
- Advocacy groups collecting data for AACR COVID-19 and Cancer conference
COVID-19 presentations from ASCO 2020
Previous reports have suggested that lung cancer patients infected with COVID-19 have worse outcomes [https://www.onclive.com/conference-coverage/aacr-2020/teravolt-data-reveal-unexpectedly-high-mortality-in-patients-with-thoracic-cancers-and-covid19] . During ASCO 2020, we heard updates from two different registry efforts focused on tracking cancer patient outcomes:
- The COVID-19 and Cancer Consortium (CCC19) registry is tracking outcomes across all cancer types. The major finding [https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31187-9/fulltext?] from this study is that patients with actively progressing cancer were five times more likely to die within 30 days of diagnosis with COVID-19 compared to patients who were in remission or had no evidence of disease. As ASCO President Dr. Howard A. Burris III states, “For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment.” These data are consistent with previous early reports and suggest that patients with active cancer are uniquely vulnerable and face worse outcomes upon infection with the virus that causes COVID-19.
- A second registry effort, Thoracic cancERs international coVid 19 cOLlaboraTion (TERAVOLT), is specifically tracking outcomes for lung cancer patients infected with COVID-19. For this study [https://www.cancernetwork.com/article/study-finds-increased-risk-death-following-chemo-use-patients-thoracic-cancer-covid-19] , 400 patients were included in the analysis, the majority of which had stage IV cancer. Among this cohort, 141 patients died from COVID-19, with 334 of the patients requiring hospitalization. Those patients receiving chemotherapy, either alone or in combination, within three months of a diagnosis of COVID-19 fared the worst, with a significantly increased risk of dying (64%) compared to those who did not receive chemotherapy.
Take home message from these studies: COVID-19 presents a unique threat to all cancer patients, especially those with lung cancer. Various international efforts are underway to understand these risks and what it means for patients and their cancer care. As states continue to reopen, it is important not to let your guard down and to maintain all the precautions you have been taking over the past few months. This virus has not gone away and it is important that you and your loved ones take appropriate steps to minimize exposure.
Advocacy groups participate in IASLC “Lung Cancer Considered” podcast
Authors of these weekly updates, including Dr. Jan Baranski, Janet Freeman-Daily, Dr. Amy Moore, and Dr. Upal Basu Roy recently participated in the International Association for the Study of Lung Cancer (IASLC) “Lung Cancer Considered” podcast. They were joined by Jill Feldman, Dr. Alice Berger, Dr. Christine Lovly, and Dr. Brendon Stiles to discuss impacts of COVID-19 on lung cancer research. Despite the obstacles created by the pandemic, lung cancer research marches on and we think you will be encouraged and inspired by the discussion. Listen here.
Advocacy groups collecting data for AACR COVID-19 and Cancer conference
In light of the COVID-19 pandemic and its impact on cancer care, AACR is convening a special conference [https://www.aacr.org/meeting/aacr-virtual-meeting-covid-19-and-cancer/] focused on the presentation of emerging data in basic, clinical, and epidemiologic research related to COVID-19 and cancer. Lung cancer patients are especially vulnerable to developing a serious case of COVID-19. In order to provide the community accurate, up-to-date, and curated scientific information on COVID-19 and cancer, lung cancer patient advocacy groups have come together to support our community through joint advocacy updates.
We need your help and your perspective! We are inviting you to participate in this 10-minute survey [https://www.surveymonkey.com/r/LC_JT_Updates] to capture your concerns about COVID-19, and whether you found this collaboration and the updates useful. The survey will close at midnight Pacific Daylight Time, Friday, June 5, 2020 to allow us to prepare abstracts for submission to the AACR “COVID-19 and Cancer” virtual meeting.
You can also copy and paste this link on your web browser to take the survey.
https://www.surveymonkey.com/r/LC_JT_Updates
The data we collect from the survey will also be shared openly across all advocacy groups once the conference is completed. Thank you for your help and for providing us your perspective.
May 18, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | May 18
As different states are relaxing shelter-at-home orders and businesses are planning to re-open, it is important to understand the true extent of COVID-19 infections: both active infections (patients who are currently infected) and past infections (patients who were infected in the past and have now recovered).
Currently, active infections are tested using a nasal swab test. The FDA also recently approved a rapid antigen detection test [https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-antigen-test-help-rapid-detection-virus-causes] to identify actively infected cases.
Past infections are identified through serological (blood) tests that detect antibodies against the SARS-CoV-2 virus.
The following infographic shows the differences between the tests used for COVID-19.
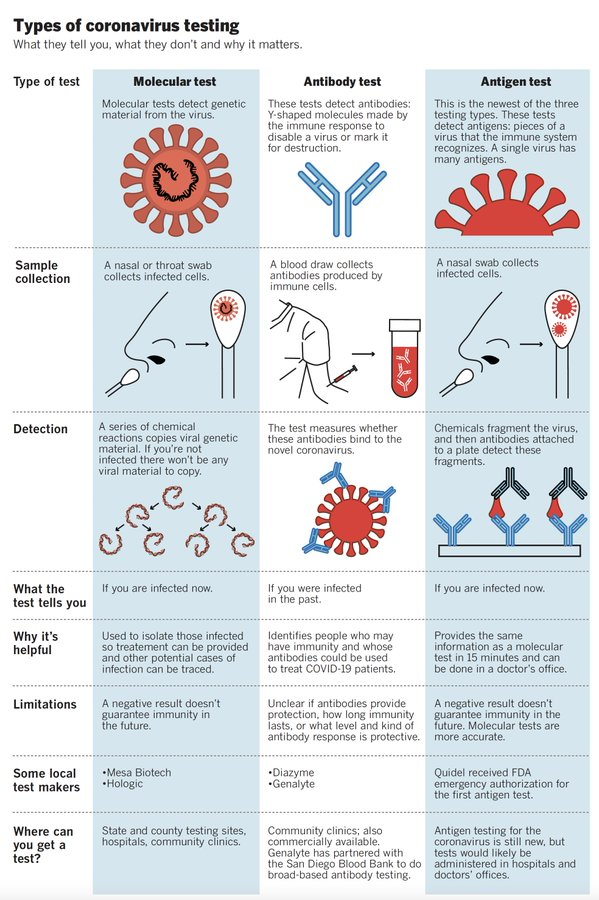
However, interpreting the results of the test may be tricky. Also, the results depend on various factors:
- Patient-specific factors: Did the patient mount a robust immune response? How long do detectable antibodies last?
- Test being used: Different antibody tests may have different sensitivity [https://www.cancer.gov/publications/dictionaries/cancer-terms/def/sensitivity] and specificity [https://www.cancer.gov/publications/dictionaries/cancer-terms/def/specificity]. We are still learning what this means for different tests and how to interpret the results.
- What a test is measuring: Some tests measure only one type of antibody (IgG) while others measure IgM and IgG. Does this mean one test is better than the other? We still do not know.
- What the test results mean: Does a positive test result mean that a person is immune to re-infection by SARS-CoV-2? If so, for how long?
For example, Amy (in the SF Bay Area) got sick on January 23, 2020 and her antibody test from May 15, 2020 was negative. Upal (in New York City) got sick on March 14, 2020 and his antibody test from May 4, 2020 was positive. Could this be because Amy didn’t produce enough antibodies? Because the antibodies decrease with time? Because the test was not done correctly? Or could it reflect differing test sensitivity?
To answer these types of questions will take time. In case you want to learn more about the issues with interpreting test results, please read the article here [https://www.cnn.com/2020/05/12/health/covid-19-antibody-test-explained-wellness/index.html].
In this week’s update, we present a short video (and transcript of the discussion) with Dr. Nicolas Vabret, Assistant Professor of Medicine, Hematology and Medical Oncology at Icahn School of Medicine at Mount Sinai. Dr. Vabret, a virologist/immunologist, answers important questions, such as:
- What type of an immune response does the body mount against SARS-CoV-2, the virus that causes COVID-19?
- How can we detect if a person is infected with SARS-CoV-2 now?
- How can we detect if a person was infected with SARS-CoV-2 in the past but has now recovered?
Resources and websites:
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute has a special website for COVID-19 and emergency preparedness: COVID-19: What People with Cancer Should Know
- We are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC).
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- The One-Two Punch: Cancer And COVID-19 (an important perspective for cancer patients)
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers
May 4, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | May 4
The authors of this weekly advocacy update are all scientists (nerds) and so before we get to this week’s update, indulge a little humor: “May the Fourth be with you!” Now back to our regularly scheduled programming:
As of May 2, 2020, the Centers for Disease Control and Prevention (CDC) reports 1,062,446 cases of COVID-19 and 62,406 COVID-19-associated deaths. Many states [https://www.wired.com/story/which-states-reopening-lockdown/] are beginning to loosen restrictions and reopen certain businesses. It is worth noting that in Georgia, the first state to reopen, the shelter in place policy has been extended [https://www.wtvm.com/2020/04/30/gov-kemp-extends-shelter-place-order-at-risk-georgians/] through June 12, 2020 for the most at-risk populations, including those over age 65, those in nursing homes or long-term care facilities, those with chronic lung disease, moderate to severe asthma, severe heart disease, class III/severe obesity, diabetes, liver disease, chronic kidney disease and undergoing dialysis, as well as those who are immunocompromised.
On Friday, May 1, 2020, the Food and Drug Administration (FDA) granted [https://www.fiercepharma.com/pharma/gilead-s-remdesivir-scores-emergency-fda-nod-covid-19-days-after-big-data-reveal] emergency use authorization for the antiviral drug, remdesivir, for the most severely ill COVID-19 patients. While this is an encouraging development, we must provide a word of caution in that we still do not have effective treatments for broad use by the general public or a vaccine.
In light of the ongoing COVID-19 pandemic, the American Association of Cancer Research (AACR) shifted its annual meeting to a virtual format and broke it into two separate events. The first was held on April 27 -28, 2020. In this week’s update, we will discuss some of the information presented during this meeting and what it means for the cancer community. In particular, we will focus on answering three questions:
- What are the implications of COVID-19 for my personal cancer treatment?
- How do I make sense of contradictory COVID-19 information?
- What are the impacts of COVID-19 on cancer research?
What are the implications of COVID-19 for my personal cancer treatment?
Since we first started providing these updates in early March 2020, there has been growing evidence that lung cancer patients infected with COVID-19 have worse outcomes. During the AACR plenary session on “COVID-19 and Cancer,” an international team led by Dr. Marina Garassino presented early data for TERAVOLT [https://www.lungcancernews.org/teravolt-thoracic-cancers-international-covid-19-collaboration/], a global registry collecting characteristics and outcomes of patients with thoracic cancers affected by COVID-19. They reported a disturbingly high mortality rate of 34.6% [https://www.onclive.com/conference-coverage/aacr-2020/teravolt-data-reveal-unexpectedly-high-mortality-in-patients-with-thoracic-cancers-and-covid19] (66/191) among patients with thoracic cancers.
As research advocates serving the lung cancer community, we recognize that these data are alarming. The immediate implications of these results fall in line with what we have been advising those who fall in high-risk groups, including lung cancer patients: continue to practice social distancing when possible, wear protective face coverings when out in public, wash hands often, and minimize travel to essential needs only (medical appointments, procuring groceries or prescriptions).
Our April 20, 2020 update discussed the increasing role for telehealth in management of patient care and our April 27, 2020 update focused on the guidelines issued by leading medical organizations and societies. Our March 30, 2020 update included impacts on clinical trials – as states begin to reopen, some trial sites are resuming enrollment. Thus, it remains imperative that you talk with your treatment team about your individual treatment plan. (See all previous weekly updates below.)
Indeed, as a result of the COVID-19 pandemic, doctors and scientists are reevaluating treatment schedules and the “usual way of doing business.” One example is the recent April 28, 2020 FDA approval [https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-new-dosing-regimen-pembrolizumab?fbclid=IwAR0ZLrZP6xY4ZZ3d-gK8mdOwqsjODC2SGPsvXoEAlczFayccmQDSZNgwhFM] for a new dosing regimen for the immunotherapy drug, pembrolizumab. This approval is based on data [https://www.abstractsonline.com/pp8/#!/9045/presentation/10751] presented at the 2020 AACR Virtual Meeting.
Dr. Jacob Sands, a leading lung cancer medical oncologist at Dana-Farber Cancer Institute, provides a nice discussion on the importance of individualized lung cancer management in the COVID-19 era here [https://www.healio.com/hematology-oncology/lung-cancer/news/online/%7B29b67c28-f77e-49e6-aa5e-185f9bcbc18e%7D/lung-cancer-management-in-the-covid-19-era-requires-an-individualized-approach].
How do I make sense of contradictory COVID-19 information?
We recommend that you follow information from trusted and medically vetted websites such as the CDC, the WHO, and the IASLC. Information on how to access these websites are included in the Resources and websites section below.
Our understanding of COVID-19 is evolving rapidly. This means that what was true a month ago may not be true under current circumstances, as doctors and scientists generate more evidence. You might hear contradictory information from different sources or at different times. As an example, the anti-malarial and autoimmune disease drug, hydroxychloroquine, was shown to have positive effect in COVID-19 patients in early studies. However, further study with more patients showed hydroxychloroquine was not as effective as it was initially thought to be, and highlighted the fact that hydroxychloroquine comes with a range of side effects that make it unsuitable for use in patients with heart issues. This is a great example of the scientific method [https://ori.hhs.gov/module-1-introduction-what-research] whereby a finding or a hypothesis changes as new information is gathered.
We also caution on how one should interpret information shared across media and press during these times. COVID-19 is a global pandemic and is affecting the oncology community everywhere in the world. Given the urgent global need for information on effective COVID-19 management, healthcare providers are sharing preliminary information as quickly as possible with the goal of learning from each other’s experiences. This means that not all information shared publicly will have the same level of evidence as formal clinical trials. The information is important and valuable, but it is not yet validated in large groups of patients.
When judging what you read from publicly available sources, we suggest you use the Evidence-Based Medicine (EBM) Pyramid as a guiding framework.
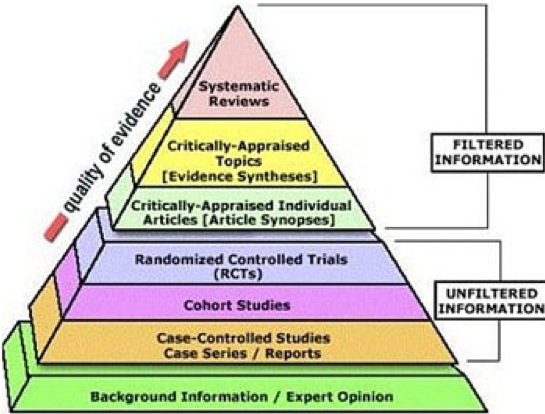
EBM Pyramid and EBM Page Generator, copyright 2006 Trustees of Dartmouth College and Yale University. All Rights Reserved. Produced by Jan Glover, David Izzo, Karen Odato and Lei Wang.
Higher quality of evidence takes longer to get published to allow for collecting larger amounts of data, statistical analysis, and scientific peer review [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4975196/]. Most of the literature currently available on COVID-19 and lung cancer are case reports and studies of a small number of patients in a few institutions. COVID-19 has not been around long enough to enable large, formal clinical trials about its impact on lung cancer treatment. If you have questions about whether published COVID-19 findings might affect your lung cancer treatment, please discuss your individual situation with your treating physician.
What are the impacts of COVID-19 on the state of academic cancer research?
Most academic research institutions, including universities and hospitals, have shut down most research labs and closed enrollment in some clinical trials to accommodate government-imposed shelter in place mandates and protect researchers’ lives. Only critical research, such as maintaining cell lines or animal models for preclinical research and some clinical trials with strong evidence of effectiveness, is being allowed to continue. These are institutionally mandated restrictions that have been put into place to protect university staff. Some researchers who are also clinicians have been deployed to assist with COVID-19-related clinical duties. Bench scientists who are not involved with clinical duties have been advised to work from home in activities such as grant and manuscript writing, and data analysis.
Funders of academic lung cancer research in the US such as the National Cancer Institute [https://www.cancer.gov/contact/emergency-preparedness/coronavirus-researchers], the Department of Defense [https://cdmrp.army.mil/about/covid-19/USAMRAA%20Supplemental%20Guidance%20on%20COVID-19_03.25.20.pdfSearch Results], and private non-profits [https://www.healthra.org/issues/covid-19/] (e.g., LUNGevity Foundation, GO2 Foundation, Lung Cancer Research Foundation, Lung Cancer Foundation of America) have all made concessions to accommodate the needs of the scientific community and best support investigators during this critical time, while trying to minimize any delays in lung cancer research. Concessions include:
- Extended deadlines for grant applications
- Allowing the use of grant funds for salaries and stipends even when researchers are not working in the laboratory
- Flexibility regarding project extensions and accommodating unanticipated costs such as loss of animals and chemicals bought for experiments
- Allowing grantees more time to report on awards after an award is completed
- Numerous flexibilities regarding expenditures of funds, such as money already spent in conferences and travels
April 27, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | April 27
As of April 24, 2020, the Centers for Disease Control and Prevention (CDC) reports 895,766 cases of COVID-19 and 50,439 COVID-19-associated deaths. As the number of cases continue to rise, the importance of maintaining social distancing and following shelter in place/quarantine orders is central to flattening the COVID-19 curve.
In this week’s update, we address how different professional societies/organizations are addressing lung cancer screening and treatment during the COVID-19 pandemic. As described in the National Academy Press [https://www.nap.edu/read/11153/chapter/9], the mission of the professional societies is primarily educational and informational. Their influence flows from their continuing and highly visible functions: to publish professional journals, to develop professional excellence, to raise public awareness, and to make awards. Through their work, they help to define and set standards for their professional fields and to promote high standards of quality through awards and other forms of recognition.
In this week’s update, we provide you with a brief summary of what different professional societies are saying about COVID-19 and lung cancer treatment. These consensus statements are a testament to the way the global oncology community is working together to ensure that lung cancer patients continue to get the best care possible. The following have been included in today’s update based on availability of information.
- CHEST – American College of Chest Physician
- ASCO – American Society of Clinical Oncology
- ESMO – European Society for Medical Oncology
- ATS – American Thoracic Society
- NCCN -National Comprehensive Cancer Network
- ASTRO – American Society for Radiation Oncology
- ESTRO – European Society for Radiotherapy & Oncology
Question 1: How are doctors managing lung cancer screening during COVID-19?
The recent consensus statement from CHEST [https://acs-cancercontrol.informz.net/acs-cancercontrol/data/images/CHEST.pdf] states that it is appropriate to defer enrollment in lung cancer screening and modify the evaluation of lung nodules due to the added risks from potential exposure to SARS-CoV-2 and the need for resource reallocation.
This means that for individuals who have not yet initiated screening, they should wait to be screened.
In those individuals where nodules are detected through a low-dose CT (LDCT) scan, the consensus statement suggests follow-up and nodule management should depend on the size of the nodule, availability of local facilities, prevalence of COVID-19 in the region, and patient-specific factors (presence of other serious health issues such as diabetes and heart disease.
Question 2: What factors are organizations/societies taking into account when deciding how to treat lung cancer patients?
According to Schrag and colleagues [https://jamanetwork.com/journals/jama/fullarticle/2764728?resultClick=1], oncology care generally falls into four categories.
- Care that is not time sensitive, can be delivered remotely, or both.
This includes survivorship and surveillance visits for patients who have completed cancer treatment (for example, a patient who has completed treatment and has no evidence of disease).
- Care that cannot be delivered remotely but for which treatment omission or delay has a marginal effect on quality or quantity of life.
The big question here is: does the risk of COVID-19 exposure outweigh the benefit of the treatment? Examples that fall into this category include: - Delaying systemic chemotherapy or reducing the number of cycles of chemotherapy for patients with advanced non-small cell lung cancer
- Delaying surgery by providing neoadjuvant chemotherapy
- Reducing the number of radiation therapy visits
- Treatment delay will have a moderate but clinically important adverse influence on quality of life or survival.
This includes using treatments that are less harsh than the original treatments, to minimize hospitalization or manage side effects during the time of COVID-19.
- Treatment that has the potential to cure and/or cannot safely be delayed. This includes treatment of small cell lung cancer.
It is important to note that recommendations should be adapted to reflect the status of the patient and available facilities.
Question 3: What are the consensus recommendations for lung cancer surgery?
ATS [https://www.annalsthoracicsurgery.org/article/S0003-4975(20)30442-2/pdf] has proposed the use of a three-phase framework to decide how to proceed with lung surgery. It defines three phases of hospital status based on:
- the prevalence of COVID-19 patents within the hospital
- availability of hospital resources, and
- the rate of change (in terms of increasing prevalence of infections and resource depletion)
Each phase has a compass statement that is meant to give additional direction on how to manage number of lung surgeries, based on perceived risk to patients and hospital staff.
| Phase 1 | Phase 2 | Phase 3 |
| • Hospital resources intact (e.g. ICU beds, ventilators, clinicians, Personal Protective Equipment available for all doctors) • COVID-19 trajectory not in rapid escalation phase | • Many COVID-19 patents • Resources limited (e.g. ICU beds, ventilators, clinicians, PPE) • COVID trajectory within hospital in rapidly escalating phase | • Hospital resources are predominately routed to COVID-19 patents • Resources critically limited/exhausted |
| Compass statement: Surgery restricted to patents whose survivorship likely to be compromised by surgical delay of 3 months | Compass statement: Surgery restricted to patents likely to have survivorship compromised if surgery not performed within next few days | Compass statement: Surgery restricted to patents likely to have survivorship compromised if surgery not performed within next few hours |
Specific treatment decisions should be made by the patient and their treating physician, keeping in mind the framework discussed in question 2.
Question 4: What are the consensus recommendations for the use of radiation for lung cancer treatment?
The ASTRO-ESTRO consensus statement [https://www.thegreenjournal.com/article/S0167-8140(20)30182-1/fulltext] follows a similar approach to the ATS statement and takes into account the local and regional scenario of the COVID-19 pandemic.
In a risk-mitigation pandemic scenario where radiotherapy resources remain available, efforts should be made not to compromise the prognosis of lung cancer patients and guideline-recommended radiation therapy should be practiced. Postponement or interruption of radiation therapy of COVID-19 positive patients should be considered to avoid exposure of cancer patients and staff to an increased risk of COVID-19 infection.
In a severe pandemic scenario characterized by reduced resources, if patients must be triaged, important factors included potential for cure, relative benefit of radiation, life expectancy, and performance status.
Specific treatment decisions should be made by the patient and their treating physician, keeping in mind the framework discussed in question 2.
Question 5: What are the consensus recommendations from medical oncology associations and professional societies?
The three professional societies/organizations (ASCO [https://www.asco.org/asco-coronavirus-information/care-individuals-cancer-during-covid-19] , ESMO [https://www.esmo.org/guidelines/lung-and-chest-tumours/lung-cancer-in-the-covid-19-era] and NCCN [https://www.nccn.org/covid-19/pdf/COVID_NSCLC.pdf] ) are aligned in their recommendations for lung cancer patients. All societies note that the risk of COVID-19 must be balanced against the risk to the patient of lung cancer progression, which in most cases still represents the highest risk of mortality in lung cancer patients. Individual clinical judgment is necessary. The recommendations provided by these societies cannot provide absolutes for alternate strategies during the COVID-19 outbreak. Specific treatment decisions should be made by the patient and their treating physician, keeping in mind the framework discussed in question 2. It is important to note that most of these recommendations would not normally be considered standard of care or optimal but are reasonable under these unusual circumstances in which minimizing visits and potential exposure has become a priority.
NCCN [https://www.nccn.org/covid-19/pdf/COVID_NSCLC.pdf] further suggests that entry points to the health care system should feature screening of patients and providers (i.e., questionnaire, temperature-based screening, standard and rapid COVID-19 testing). If resources are sufficient, screening of visitors who can accompany patients is reasonable, although many institutions have visitation restrictions to facilitate social distancing.
Question 6: How do these changes in lung cancer care impact shared decision-making?
Shared decision-making is [https://www.healthit.gov/sites/default/files/nlc_shared_decision_making_fact_sheet.pdf] a process in which patients and doctors work together to make decisions and select tests, treatments, and care plans based on clinical evidence that balances risks and expected outcomes with what individual patient value. The consensus statements from all the professional societies urge doctors to have candid discussions with their patients and to take into account patient preferences and values when making decisions for screening and treatment.
Question 7: Should lung cancer treatment be modified if patients also have COVID-19?
Physicians don’t have much data to help guide treatment decisions for lung cancer patients who also have COVID-19. To gather this data, the global lung cancer community has come together to develop the TERAVOLT registry [https://www.esmo.org/content/download/284671/5626123/1]. The registry is collecting information on patients with thoracic cancer infected with COVID-19 regardless of therapies administered. More than 100 physicians worldwide are participating, and the number is growing. Currently, patients cannot deposit their data into the registry themselves. If you have or had a confirmed case of COVID-19 and would like your data included in the registry, talk to your doctor about joining the TERAVOLT registry.
April 20, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | April 20
As of April 18, 2020, the Centers for Disease Control and Prevention (CDC) reports 661,712 cases of COVID-19 and 33,049 COVID-19-associated deaths. A recent study conducted by researchers at Stanford University suggests that this number is an underrepresentation of the total number of infected individuals. We must urge caution with interpretation of this study, which is still undergoing peer-review. Some are interpreting the findings to mean we should accelerate loosening of social distancing policies across the country. However, researchers still do not know if the presence of antibodies confers protection or how long such immunity might last. Further, in the absence of any effective therapeutics or a vaccine, local flare-ups are still a risk, as has been witnessed in other countries. Social distancing is working and should be maintained for now.
In this week’s update, we address the following important topics:
Role of telehealth in the era of COVID-19
- What is telehealth?
- How is the use of telehealth changing during the COVID-19 pandemic?
- What are some of the barriers to broad uptake of telehealth during the current crisis?
- How do I know if I am eligible to obtain telehealth services?
Impact of COVID-19 on lung cancer clinical trials
- How is the FDA allowing the use of telehealth for lung cancer clinical trials?
- If a patient is receiving their drug through a pharmacy at the clinical trial site, can they now receive the drug through home delivery without having to change the protocol?
- If a patient is receiving a drug given through infusion, can they now receive the clinical trial treatment through home infusion?
Role of telehealth in the era of COVID-19
1. What is telehealth?
The terms “telehealth” and “telemedicine” are used interchangeably to describe using telecommunications technologies to deliver health care. It includes a variety of services that deliver health care, public health, and health education, and ranges from methods as simple as telephone calls and email to live video, mobile apps, remote patient monitoring and uploading scan images. The Center for Connected Health Policy (CCHP) provides an excellent overview of telehealth here.
2. How is the use of telehealth changing during the COVID-19 pandemic?
In light of the COVID-19 pandemic, federal and state policies are rapidly adapting to allow for greater utilization of telehealth services. The Centers for Medicare & Medicaid Services (CMS) have created some useful fact sheets highlighting various policy changes, including this one summarizing Medicare telemedicine services and this one addressing sweeping regulatory changes to meet patients’ needs during this time. Private health insurance companies are also modifying their policies to enable greater use of telehealth.
CCHP is maintaining an updated list of COVID-19 telehealth coverage policies.
3. What are some of the barriers to broad uptake of telehealth during the current crisis?
The challenge with adapting telehealth policies in real-time to address an unfolding and unprecedented public health crisis is that, in a pre-COVID-19 world, federal and state policies varied widely in how telehealth services were provided and covered.
Most of the current challenges relate to regulatory and reimbursement issues, including licensure requirements. Even as the popularity of telehealth among patients grows, private healthcare payers have been slow to embrace the technology. The Federation of State Medical Boards is maintaining an updated list of states currently waiving telehealth licensure requirements.
The “digital divide” is also a barrier to accessing telehealth. Patients who are elderly, in areas with poor Internet or cellular coverage, or economically disadvantaged, may not be able to access the technology necessary to telehealth.
4. How do I know if I am eligible to obtain telehealth services? What can I do to ensure broader access?
Again, laws vary by state regarding how telehealth is being implemented and what health insurance companies and providers can do. Review your health insurance plan benefits and policies frequently to learn how they may be changing. This CCHP list of COVID-19 state actions may also be helpful.
CCHP also monitors state and federal telehealth legislation to provide a clear overview of policy across the nation. As a citizen, you can monitor legislation that has been introduced in your state and testify to show your support or opposition. You can call your legislators to ensure your needs are being heard.
For a great overview and more in-depth discussion on all of these points on telehealth, please check out GO2 Foundation for Lung Cancer’s Rapid Response Living Room from April 14, 2020, featuring Dr. Joelle Fathi.
Impact of COVID-19 on lung cancer clinical trials
The United States Food and Drug Administration(FDA) has issued guidance to clinical trial sponsors (pharmaceutical companies and government agencies), institutional review boards (IRBs), and researchers on how to adapt lung cancer clinical trials in the era of COVID-19. The FDA emphasizes that patients’ safety should be at the forefront of considerations at all times. Below, we answer three important questions for patients (and their caregivers).
1. How is the FDA allowing the use of telehealth for lung cancer clinical trials?
The FDA allows for changes to be made to the clinical trial protocol without prior FDA review or approval if the change is intended to protect the life and well-being of the patient. Therefore, changes in protocol conduct necessary to immediately assure patient safety, such as use of telehealth for safety monitoring instead of on-site visits, can be immediately implemented once the new protocol has been approved by an IRB. The FDA can then be subsequently notified. It is important to note that the consult is just one part of patient safety monitoring. The patient’s clinical trial team and the clinical trial sponsor will also need to have a clear plan in place to ensure that patient safety is prioritized in case routine monitoring such as blood tests and heart function exams are unable to be conducted.
2. If a patient is receiving their drug through a pharmacy at the clinical trial site, can they now receive the drug through home delivery?
The FDA understands that there may be a risk of exposure to SARS-CoV-2 when a patient visits a clinical trial site. In case a patient is receiving their drug (such as a targeted therapy pill) through their clinical trial site pharmacy, the clinical trial sponsor now has the option of directly mailing the drug to the patient’s home as long as the following conditions are met:
- The patient already takes the pill at home as part of the trial protocol
- The shipment of the drug to the patient’s home does not affect the chemical nature of the drug
- The sponsor keeps a clear track of number of pills shipped to the patient’s home and is able to share this information with the FDA when asked
3. If a patient is receiving a drug given through infusion, can they now receive the clinical trial treatment through home infusion?
This is an extremely important question for the lung cancer community — where clinical trials often require an infusion of a chemotherapy, an immunotherapy, an angiogenesis inhibitor, or a combination of the above.
The FDA understands and appreciates that a patient may be exposed to SARS-CoV-2 when they travel to their routine clinical trial infusion center. Therefore, the FDA is open to alternative sites for administration (e.g., home nursing or alternative sites closer to a patient’s home where the infusion is given by trained medical personnel who are not part of the study team). The ultimate decision to allow this switch to home infusion or local infusion is based on the following criteria:
- The shipment of the drug to the local infusion center or to the patient’s home does not affect the chemical nature of the drug
- The sponsor keeps a clear track of the amount of shipped to the patient’s home and is able to share this information with the FDA when asked
Another option is delaying or discontinuing infusion for a period of time while the patient continues to be on the study. This decision needs to be made jointly by the clinical trial team and the patient.
Note: The ultimate decision on whether to allow a home infusion or local infusion is highly dependent on the drug being tested. Some infusions cannot be given at a local infusion center or through home infusion. Examples include drugs that require ability to manage potential infusion reactions with specific medication, or treatments such as gene therapy or cell therapy that require exacting handling procedures and patient monitoring.
LUNGevity Foundation recently conducted an Oncology Center of Excellence (OCE) listening session with FDA leadership and lung cancer patients. Stay tuned for the recorded webinar that can be accessed here.
April 13, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | April 13
As of April 11, 2020, the Centers for Disease Control and Prevention (CDC) reports 492,416 cases of COVID-19 and 18,559 COVID-19-associated deaths. The United States now has the highest number of confirmed COVID-19 cases in the world. These numbers may be an underestimation of the true burden of the disease due to lack of testing and a high proportion of asymptomatic yet infectious individuals.
We urge everyone to continue to social distance: it is working!
Washington and California quickly imposed state-wide social distancing through stringent shelter-in-place or Stay at Home policies once deaths occurred within their borders. The graph below shows that COVID-19 deaths are still increasing in these two states, but not as fast as in other states. Deaths in New York State are now doubling every 3 days (when last week it was every 2 days), and in Washington State and some California counties deaths are doubling only every week.
The most up-to-date version of the graph below can be accessed from the New York Times website here.
In this week’s update, we answer the following important questions:
- What types of treatments are available for COVID-19?
- If I suspect I have COVID-19, what should I do?
- If I had COVID-19 and have now recovered, will I become immune to SARS-CoV-2?
- Can I find out if I was naturally infected with SARS-CoV-2, the virus that causes COVID-19, and have developed immunity?
- How can I tell if information I read about COVID-19 is reliable?
(1) What types of treatments or vaccines are available for COVID-19?
If you or your loved one (caregiver) has a confirmed diagnosis of COVID-19, your/their healthcare provider will be the right person to decide what type of treatment is useful. Please do not treat COVID-19-related symptoms without consulting your healthcare provider. Do not try to procure or hoard medications such as hydroxychloroquine that are approved for use in other conditions, as this is creating a shortage of these medications for patients with conditions for which the drugs are already approved.
Currently, no specific treatment or vaccine is available for COVID-19. This continues to be an area of intense investigation. Some of the drugs described below may be available to COVID-19 through compassionate access. This does not mean a drug is approved for the treatment of COVID-19. It is important to keep in mind that properly controlled clinical trials take a long time to conduct.
Treatment: Various compounds such as remdesivir and hydroxychloroquine are being studied for the treatment of COVID-19.
Remdesivir is an anti-viral drug. It has been shown to be active against a broad group of viruses such as filoviruses (cause of Ebola) and coronaviruses (e.g., SARS-CoV and MERS-CoV). SARS-CoV is the cause of SARS and MERS-CoV is the cause of Middle East Respiratory Syndrome. These two viruses are related to SARS-CoV-2, the cause of COVID-19. Remdesivir has shown to protect against infection and treat infection caused by these coronaviruses in pre-clinical models (experiments involving animal models of virus infection). A recent publication in the New England Journal of Medicine shows that remdesivir given to hospitalized COVID-19 patients helped improve symptoms. Though promising, it is important to note that this study included a small sample of patients. Randomized clinical studies with appropriate controls are necessary before remdesivir becomes approved for use with all COVID-19 patients.
Hydroxychloroquine is a drug used to treat malaria and auto-immune disorders such as rheumatoid arthritis. It is known by the name Plaquenil in the US. Currently, evidence suggesting the successful use of hydroxychloroquine in COVID-19 patients is limited, and mostly anecdotal. Clinical trials studying the effect of hydroxychloroquine in the treatment of COVID-19 are ongoing. Despite the President’s ongoing endorsement of hydroxychloroquine as an effective treatment for COVID-19, concerns about its cardiac toxicities are growing, especially for people with underlying cardiovascular disease. Recent guidance issued by leading heart disease organizations recommends caution when treating COVID-19 with the combination of hydroxychloroquine and the antibiotic azithromycin in certain patients.
Vaccines: Historically, vaccines against viruses have been very effective in eradication of common viral diseases such as smallpox and polio. From an individual perspective, vaccination prevents or modifies the severity of a disease. From a public health perspective, a vaccine eliminates or eradicates a disease from the population (for example, smallpox). Since the SARS epidemic in 2003, several companies and institutions have already developed platforms for vaccine development against coronaviruses, which is a family of viruses that includes SARS-CoV-2, the causative agent of COVID-19. On January 23, 2020, the Coalition for Epidemic Preparedness Innovations (CEPI) was the first to announce funding of $12.5 million to develop vaccines against SARS-CoV-2 by three companies. Since then, both philanthropic and industry efforts have been mobilized for the development of a vaccine against SARS-CoV-2. As of April 9, 2020, there are close to 80 vaccine development efforts. It is important to keep in mind that vaccine development is a lengthy process and best-case estimates suggest that it will take a minimum of 18 months to deliver an effective vaccine against SARS-CoV-2. Even once a candidate vaccine is identified, it must be manufactured at scale for a global population. To speed these efforts, the Gates Foundation has announced that it is supporting construction of factories to pursue development of seven vaccine candidates.
Other treatments: Recently, the Bill & Melinda Gates Foundation also announced a $125 million partnership with Wellcome and Mastercard to accelerate the development of new drugs to treat SARS-CoV-2 infection.
(2) If I suspect I have COVID-19, what should I do?
If you (as a patient) or a loved one (a caregiver) exhibits the symptoms of COVID-19 (including but not limited to fever, dry cough, shortness of breath), immediately follow self-quarantine procedures. Immediately contact your healthcare provider (could be your primary care physician) about whether you need to get tested. Your healthcare provider will be able to guide you on next steps. The CDC and the Association of Public Health Laboratories maintain a list of laboratories that are currently conducting COVID-19 tests.
Please remember that you may not have symptoms even if you are infected with SARS-CoV-2. Social distancing is the only way to avoid getting infected with the virus. As a caregiver, please take extra precautions when you need to leave your house or apartment.
(3) If I had COVID-19 and have now recovered, will I become immune to SARS-CoV-2?
The answer is – we do not know. When an individual is infected with a pathogen, such as bacteria or a virus, typically the body mounts an immune response. This is true even in individuals who remain asymptomatic. The immediate immune response ensures that the pathogen is eliminated from the body, and immunological memory (the basis of vaccination) ensures that if the body encounters the pathogen in the future, the immune system is ready to fight it off.
In the case of SARS-CoV-2, based on experience with other coronaviruses such as SARS-CoV and MERS-CoV, we believe that development of immunity will be highly individual-specific. Some individuals may develop high amounts of antibodies against SARS-CoV-2 while others may not. Furthermore, it is not currently clear whether a one-time infection with SARS-CoV-2 confers lifetime immunity against the virus. Scientists are still evaluating what level of immunity is needed to confer protection against future infections with SARS-CoV-2.
(4) Can I find out if I was naturally infected with COVID-19 and have developed immunity?
The answer as of April 13, 2020 is – we do not know. In order to check if an individual has been infected with a pathogen, healthcare providers can conduct blood tests (also known as serological tests) to check for indicators of immunity. For example, if you received the MMR vaccine, the doctor can check if you have immunity against measles, mumps, and rubella by looking at antibody titers in your blood.
In the case of SARS-CoV-2, the goal is to develop such a blood test that will help identify:
- Which individuals have been naturally infected with SARS-CoV-2 and have recovered from the infection (even those individuals who were asymptomatic)
- Which individuals have developed immunity against SARS-CoV-2 after they received a vaccine when the vaccine becomes available
Currently, scientists are studying which antigens of the SARS-CoV-2 will be the most effective in generating immunity. Several groups are developing blood tests, and one test has been FDA approved (but is not yet publicly available). There is hope that such tests will become available within the next few months (and probably sooner in some places), and will be useful in guiding how quickly normal activities can resume.
(5) How can I tell if information I read about COVID-19 is reliable?
In the era of COVID-19 when information is evolving rapidly — with new information being available daily — it is important to depend on trusted and reputable sources of information. You will encounter several sources of information such as:
- Print (newspapers such as New York Times which also have online versions)
- Online-only platforms such as Slate and Buzzfeed
- Journal article websites
- Personal blogs and stories
It is incredibly important to verify sources of your information to ensure that we are not spreading false information which can not only cause unnecessary panic but can be downright harmful (such as misinformation regarding drugs). A good resource that provides guidelines to evaluate health information can be found here.
April 6, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | April 6
The number of confirmed cases of COVID-19 continues to grow in the US. As of April 5, 2020, there are 312,089 confirmed cases of COVID-19 in the US. These numbers may be an underestimation of the true burden of the disease due to lack of testing and a high proportion of asymptomatic yet infectious individuals.
To get a real-time understanding of the cases in the US, you can access the map through The New York Times.
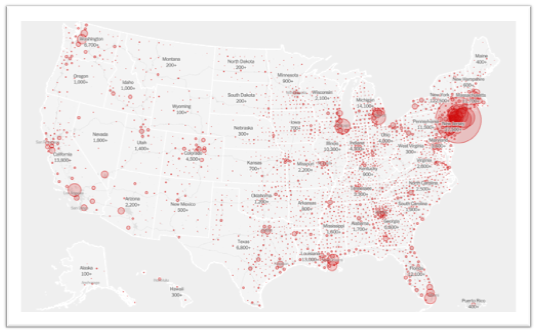
In this week’s update, we answer the following important questions:
- Is social distancing working?
- Do I continue to social distance?
- Do I leave my city to go to someplace safe — such as a rural area?
- Should I use homemade masks to protect myself?
- Can I travel within the United States?
(1) Is social distancing working?
YES, IT IS! Washington and California quickly imposed state-wide social distancing through stringent shelter-in-place or Stay at Home policies once deaths occurred within their borders. The graph below shows that COVID-19 deaths are still increasing in these two states, but not as fast as in other states. Deaths in New York State are doubling every 2 days, but in Washington State deaths are doubling only every 7 days.
The most up-to-date version of the graph below can be accessed from the New York Times website here.
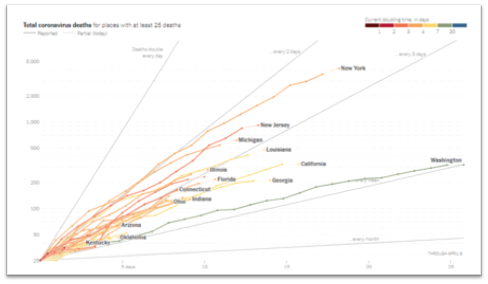
The CDC continues to recommend social distancingto help decrease transmission of COVID-19 within the community. Social distancing measures, such as cancelling public gatherings and avoiding crowds, can slow the spread of the virus and spread cases out over a longer period of time, which can help hospitals provide care while avoiding being overwhelmed by patients. Social distancing helps “flatten the curve” in the spread of an infectious disease.
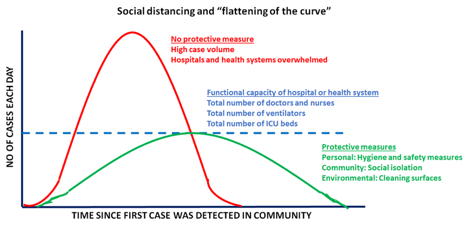
This is especially critical because hospitals and health systems are working at full capacity. Flattening the curve ensures that systems are functional and people who require care the most can get the attention they need. The CDC recommends a distance of 6 feet or 2 meters as the minimum distance between individuals as part of COVID-19 mitigation strategy.
We encourage you to check out CDC’s COVID-19 community mitigation strategies here.
(2) Do I continue to social distance?
YES, social distancing is currently the only known public health measure that can protect us from SARS-CoV-2. Social distancing minimizes risk of exposure to the virus. It is important to keep in mind that social distancing is NOT the same as social isolation. We encourage you to keep in touch with all your family, friends, and loved ones through phone and video conversations. Just because we are social distancing doesn’t mean we have to be socially isolated.
Social distancing is different from self-quarantine. Self-quarantine should be practiced by people who have been exposed to SARS-CoV-2 and who are at risk for coming down with COVID-19. It involves:
- Using standard hygiene such as frequently washing your hands and not touching your face
- Not sharing things like towels and utensils with other people who share the same household
- Staying at home and not having any visitors
- Staying at least 6 feet away from other people in your household
You do not need to have symptoms of COVID-19 to self-quarantine. Caregivers who have travelled or are leaving their homes frequently to run errands may choose to self-quarantine especially in COVID-19 hot zones.
(3) Do I leave my city to go to someplace safe – such as a rural area?
NO! You should not be leaving your urban apartment or home and move to a rural area for the following reasons:
- You do not know if you or a loved one has been infected with SARS-CoV-2. An infected individual might not have any symptoms but still can continue to infect others
- You may not have access to your regular doctors and healthcare in a rural setting
- Small rural areas are not equipped to handle an influx of people. They may not have adequate number of grocery and produce stores. More importantly, they may not have adequate medical care facilities should there be an emergency
Do not indulge in “disaster gentrification” for your own safety, the safety of your loved ones, and that of other community members. It is our duty to keep rural communities insulated from this disease as much as possible.
(4) Should I use homemade masks to protect myself?
YES! The CDC reports that a significant portion of individuals with COVID-19 lack symptoms (“asymptomatic”) and that even those who eventually develop symptoms (“pre-symptomatic”) can transmit the virus to others before showing symptoms. This means that the virus can spread between people interacting in close proximity—for example, speaking, coughing, or sneezing—even if those people are not exhibiting symptoms. In light of this new evidence, CDC recommends wearing cloth face coverings in public settings where other social distancing measures are difficult to maintain (e.g., grocery stores and pharmacies) especially in areas of significant community-based transmission.
Masks should be worn in conjunction with maintaining adequate social distancing. It is also important to keep in mind that these face masks are not surgical masks or N95 masks (to be used primarily by healthcare works as part of personal protective equipment).
(5) Should I travel within the US?
- Is COVID-19 present in your home community? If so, avoid traveling to avoid spreading the virus. You might be required to enter quarantine at your destination.
- Is COVID-19 spreading in the area where I am travelling to?
- Will you or your travel companion(s) be in close contact with others during your trip?
- Are you or your travel companion(s) more likely to get severe illness if you get COVID-19?
- Do you have a plan for taking time off from work in case you are told to stay home for 14 days for self-monitoring or if you get sick with COVID-19?
- Do you live with someone who is older or has a serious, chronic medical condition (especially important for caregivers)?
Though the CDC does not typically make suggestions about domestic travel, they highly recommend avoiding all non-essential travel within the US. Several cities and states already have a shelter-in-place order. Points to keep in mind should you need to consider domestic travel (from the CDC):
March 30, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | March 30
As of March 30, 2020, cases of the virus surge in countries around the world. The United States now has the highest number of COVID-19 cases globally. The CDC has issued a travel advisory for the New York tri-state area, which has the highest number of cases in the country.
In this week’s update, we discuss lung cancer treatment and clinical trials in the era of COVID-19. The information presented in this update, though current, is a work-in-progress built on very little data. Implementation across institutions and trial sites will vary based on availability of resources and healthcare workers.
Also, please don’t forget to check out the Resources list.
Lung cancer treatment and COVID-19
The oncology community is currently balancing treatment decisions for lung cancer patients, in light of the COVID-19 pandemic. Two factors are being used to decide what’s best for our patients:
- Whether a delay in cancer diagnostic tests or treatment presents more risk than potential COVID-19 exposure in the clinic
- Whether a difference in treatment approach can help reduce clinic visits and interactions with others
The CDC continues to recommend social distancing to help decrease transmission of COVID-19 within the community. Social distancing measures, such as cancelling public gatherings and avoiding crowds, can slow the spread of the virus and spread cases out over a longer period of time, which can help hospitals provide care while avoiding being overwhelmed by patients. Social distancing helps “flatten the curve” in the spread of an infectious disease. This is especially critical because hospitals and health systems are working at full capacity. Flattening the curve ensures that systems are functional and people who require care the most can get the attention they need. The CDC recommends a distance of 6 feet or 2 meters as the minimum distance between individuals as part of COVID-19 mitigation strategy.
Currently, lung cancer patients may need to engage with the oncology care system for the following reasons:
- clinic visits
- tissue and liquid biopsies
- surgical procedures
- infusion sessions for chemotherapy or immunotherapy (or both)
- refill targeted therapy drugs
- radiation treatments
- hospital admissions
- blood draws for laboratory tests, and
- imaging tests to check if treatments are working
Also, family members may sometimes accompany patients when they are visiting their doctors.
Recent studies out of China suggest that hospital admissions and repeated clinic visits increase the risk of COVID-19 exposure for patients. Further, a JAMA Oncology study reported that the infection risk for cancer patients in a tertiary care institution was 2-fold higher than the cumulative incidence observed in the city of Wuhan over the same time period. In light of these data and the rapidly evolving COVID-19 pandemic, the oncology community has come up with the following suggestions for cancer treatment. Please be advised that these recommendations are subject to change.
Small Cell Lung Cancer (SCLC):
If you have a confirmed diagnosis, you may not wish to delay treatment (such as chemotherapy and radiation). You and your doctor should discuss what’s right for you.
Early stage non-small cell lung cancer (NSCLC) (Stage I to IIIB):
- If you have already had surgery, your doctor may decide to not start with adjuvant chemotherapy and/or radiation.
- If you have not yet had surgery, you and your doctor may decide to wait on the surgery or your doctor may suggest stereotactic body radiation therapy (SBRT).
- If you are currently having chemo-radiation, your doctor may decide to continue with your treatment or wait on additional treatment.
Advanced stage non-small cell lung cancer (NSCLC) (Stage IIIC-IV):
- If you are on a targeted therapy (pill), you may continue with your treatment as planned. Make sure to check with your doctor and pharmacist to ensure an adequate supply of your cancer medication.
- If you are already on immunotherapy or chemotherapy, your doctor may decide to continue with your treatment, space out infusions, or postpone treatment. They may decide to have you receive infusions at your local clinic or even home infusion, as needed.
- If you are already undergoing radiation therapy, your doctor may choose to hold off on additional treatment, reduce the number of treatments, or keep you on treatment as planned, based on your individual health situation.
Several recent forums have discussed the management of lung cancer during the COVID-19 pandemic. Topics that are currently being addressed by lung cancer providers/thought leaders include:
- How to determine whether pneumonitis is resulting from checkpoint inhibitor or COVID- 19 infection
- Should immunotherapy be withheld from patients whose tumors do not have known driver mutations (as determined by molecular testing)?
- Spacing out or postponing infusions for patients on pemetrexed or immunotherapy maintenance
- Reducing the number of fractions used in radiation therapy
- Uncertainty regarding how COVID-19 treatments in clinical trials (such as remdesivir and hydroxychloroquine) may interact with immunotherapy drugs and tyrosine kinase inhibitors
- The growing role for liquid biopsies in places where surgical biopsies are not currently practical (use of mobile phlebotomy too)
- Challenges of spacing out chemotherapy schedules in light of current reimbursement
- Growing role for telemedicine (effective for managing patients but loss of doctorpatient bond)
- Educating others on their care teams to overcome lung cancer nihilism and stigma
All treatment decisions should be made jointly by you and your doctor. Do not change your treatment plan or doctor’s visit without consulting your doctor first.
Telehealth or remote consults may be an option for checking in with your doctor. Also, there may be the option to be referred to a “COVID-19-free” hospital or treatment center.
Clinical trials and COVID-19
Clinical trials continue to be a source of life-saving therapies for lung cancer patients. The COVID-19 pandemic has affected the conduct of clinical trials due to the following reasons:
- Questions related to safety of patients traveling to trial sites and undergoing trial-related procedures
- Potential shortage of healthcare providers to conduct trial-related activities
- Interruptions to the supply chain of the drug(s) being tested
The US Food and Drug Administration has recently issued guidance to help clinical trial sponsors figure out the best approaches to ensure that trials can proceed within resource-constrained settings. A clinical trial sponsor in this case is defined as any entity (for example, a pharmaceutical company) involved in the development and testing of drugs and other interventions in clinical trials. Below we have summarized key points from the FDA guidance that are important from the patient perspective.
For clinical trials that are already ongoing:
Sponsors should consider each circumstance, assess the potential impact on the safety of trial, and modify study conduct accordingly. Decisions regarding this could include continuing trial recruitment, continuing use of the new drug(s) for patients already involved in the trial, and the need to change patient monitoring schedules throughout the trial. Clinical trial participants should be kept updated on any changes that a sponsor decides to implement.
- Sponsors, doctors involved in the trials, and Institutional Review Boards (IRBs) may decide that the protection of a patient’s safety, welfare, and rights would be best served by continuing or by discontinuing use of the investigational product or participation in the trial. However, such decisions will depend on the specific circumstances of the clinical trial and the patients enrolled.
- Given that trial participants may be unable to come to investigational sites due to protocol-specified visits, sponsors should assess whether alternative methods for safety evaluations could be implemented when necessary and feasible. Additionally, in deciding to continue or discontinue use or administration of the new drug(s), sponsors should consider whether the safety of participants can be ensured by implementing the alternative approach of monitoring such as local scans and blood tests. Sometimes, patients may require additional safety monitoring.
Several sponsors already have different measures in place to allow conduct of clinical trials and avoid as much disruption as possible, such as:
- Allowing patients to have blood draws and CT scans at local cancer centers and clinics
- Shipping drug supplies to patients, especially for targeted therapy (pills) trials
- Remote consent
- Mobile phlebotomy
If you are part of a clinical trial, we recommend you discuss your trial participation immediately with your research team. If you were considering enrolling in a clinical trial, you may want to discuss with your treating physician what options are available for you. Any decision about trial participation should be made jointly by you and your healthcare team.
March 23, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | March 23
As cases of the virus surge in countries around the world, with Italy being particularly hard hit, many nations are taking extreme steps to mitigate the outbreak, including whole country lockdowns. Here in the United States, the President declared a national emergency on March 13, 2020. Several states have declared shelter-in-place to minimize non-essential activities and mitigate transmission. President Trump has declared California, New York State, and Washington State to be major disaster areas.
In this week’s update, we discuss the following topics related to COVID-19:
Origin of SARS-CoV-2
SARS-CoV-2 is the seventh coronavirus known to infect humans. SARS-CoV, MERS-CoV, and SARS-CoV-2 can cause severe disease, whereas HKU1, NL63, OC43 and 229E are associated with mild symptoms. There has been a lot of speculation on the origin of SARS-CoV-2. Scientists have now sequenced the genetic material of the virus isolated from different patients. These sequencing results clearly establish that SARS-CoV-2 is not a genetically engineered virus, meaning it is not manmade.1
The researchers provide two scenarios for the origin of SARS-CoV-2. In one scenario, the virus evolved to its current pathogenic (disease-causing) state through natural selection in a non-human host and then jumped to humans. This is how previous coronavirus outbreaks have emerged, with humans contracting the virus after direct exposure to civets (SARS) and camels (MERS). The researchers proposed bats as the most likely reservoir for SARS-CoV-2 as it is very similar to a bat coronavirus. In the other proposed scenario, a non-pathogenic version of the virus jumped from an animal host into humans and then evolved to its current pathogenic state within the human population. For instance, some coronaviruses from pangolins, armadillo-like mammals found in Asia and Africa, have similarities to SARS-CoV-2. A coronavirus from a pangolin could possibly have been transmitted to a human, either directly or through an intermediary host such as civets or ferrets.
Which age groups have severe responses to COVID-19?
Initial data on COVID-19 suggested that when stratified by age, the elderly were the most likely to develop a more severe form of COVID-19. Recent data released by the CDC demonstrated that this is not the case anymore. As shown in the figure below, almost all age groups are susceptible to a serious form of COVID-19 that requires hospitalization.2
This is especially important to keep in mind given that younger people have been more resistant to social distancing.
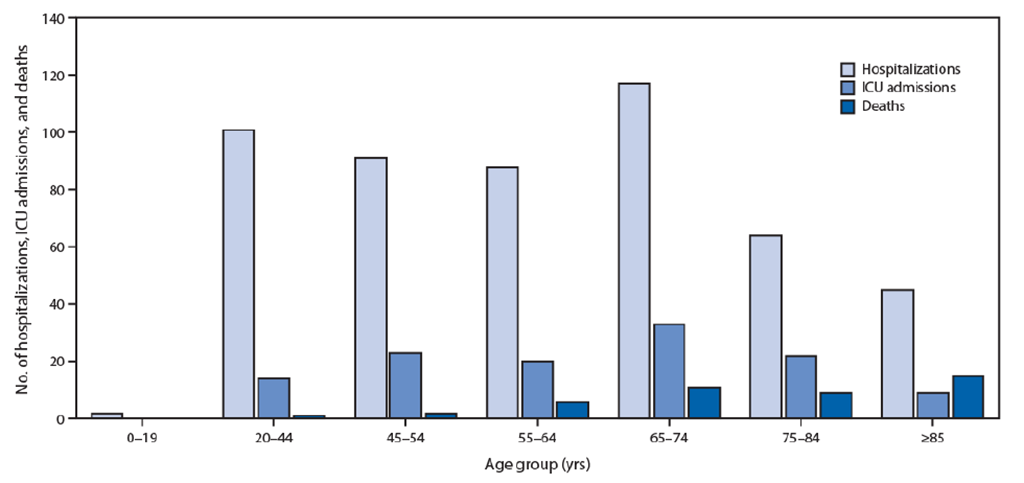
How long does SARS-CoV-2 survive outside the body?
A recent study found that the SARS-CoV-2 virus (that causes COVID-19) can survive up to four hours on copper, up to 24 hours on cardboard, and up to two to three days on plastic and stainless steel. The researchers also found that this virus can hang out as droplets in the air for up to three hours before they fall. But most often they will fall more quickly.3
The researchers were able to detect viable viral particles for at least 72 hours on the four surfaces studied. This suggests that transmission of SARS-Cov-2 is possible through aerosols and fomites (solid objects and surfaces that are able to carry pathogens and transmit infections).
We recommend that after you bring articles into your home, you do the following:
- Wash your hands after carrying delivered items into your home.
- After accepting a package that’s in a cardboard container, put it aside or in the garage and let it sit for a day or two before opening (if possible).
- After opening a package, wipe down all articles that have solid surfaces with chlorine wipes or disinfect with an alcohol-based solution.
- At this time, there is no guidance on how to disinfect edible items such as fruits and vegetables.
- Follow cleaning and disinfecting procedures listed on the coronavirus.gov website
Community transmission of SARS-CoV-2 by asymptomatic individuals
Data from initial cases of COVID-19 suggested that most transmissions were occurring through individuals who showed signs and symptoms of COVID-19. This is however not the case. It is now estimated that as many as 31% of new COVID-19 infections are being caused as a result of transmission through asymptomatic individuals – those who have been infected with SARS-CoV-2 but don’t shown signs and symptoms of the disease.4 This is an especially important aspect of SARS-CoV-2 transmission and reinforces why we need to practice stringent social distancing to flatten the curve.
COVID-19 patients may present with non-respiratory symptoms even before they have respiratory symptoms
Individuals infected with SARS-CoV-2 may present with gastrointestinal symptoms such as anorexia (83.8%), diarrhea (29.3%), vomiting (0.8%), and abdominal pain (0.4%).5 These gastrointestinal symptoms may show up even before respiratory symptoms of COVID-19. Furthermore, a small sample of patients presented with only gastrointestinal symptoms. If you have unexplained gastrointestinal issues, we suggest that you talk to your doctor promptly. Also, conjunctivitis may be present in a small subset of patients as well. 6
Prepare your legal documents
Given the uncertainty over availability of medical care during the COVID-19 emergency, we suggest everyone review their legal documents and ensure they have a current Durable Power of Attorney and Advance Directive. This virus can progress very rapidly and seriously interfere with breathing, which means you cannot be certain that you will be able to make your wishes known verbally if you get severely ill. Discuss your wishes with your family and ensure everyone knows where to find these important documents.
If you haven’t completed these legal documents, some estate planning attorneys may be willing to help prepare and witness them via video conferencing so that you do not have to leave your home.
If you are not sure how to get started, please review the resources available at https://theconversationproject.org/
Can I take ibuprofen when I have COVID-19?
Short answer, yes. Long answer: we’re not sure.
On March 18, the World Health Organization (WHO) posted an article suggesting that patients who have COVID-19 avoid taking ibuprofen, based on observations of patients in France.7 However, later the same day, WHO changed their stance and said patients who have COVID-19 should not avoid taking ibuprofen.8
This is a good example of how quickly information is evolving during this pandemic. It’s difficult for doctors to know whether to act on information that is based on the experience of only a few (or even one) patient.
References
- Anderson K, Rambaut A, Lipkin W, Holmes E, Garry R. The proximal origin of SARS-CoV-2. Nature Medicine. 2020.
- CDC. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020.
- Nishiura H, Kobayashi T, Suzuki A, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis. 2020.
- Pan L, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. American Journal of Gastroenterology. 2020.
- AAO. Alert: Important coronavirus updates for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Published 2020. Accessed March 23, 2020.
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020.
- ScienceAlert. Updated: WHO Now Doesn’t Recommend Avoiding Ibuprofen For COVID-19 Symptoms. https://www.sciencealert.com/who-recommends-to-avoid-taking-ibuprofen-for-covid-19-symptoms/amp?fbclid=IwAR0f9eZt8u9s_xfiY06bJ0Sei2NasHQj_b_eosKGjBeJiJXi5LXQV3EIj7w. Published 2020. Accessed March 23, 2020.
March 16, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | March 16
The World Health Organization officially declared the COVID-19 outbreak a pandemic on March 11, 2020. As cases of the virus surge in countries around the world, with Italy being particularly hard hit, many nations are taking extreme steps to mitigate the outbreak, including whole country lockdowns. Here in the United States, the President declared a national emergency on March 13, 2020.
In this week’s update, we discuss four important topics related to COVID-19.
1. Social distancing and why it matters for COVID-19
COVID-19 is caused by the virus, SARS-CoV-2. Individuals infected with SARS-CoV-2 appear to shed the virus from their respiratory tract (e.g., when coughing) even when symptoms may be very minor. Individuals infected with SARS-CoV-2 appear to shed the virus from their respiratory tract during the prodromal period.1 The prodromal period is part of an infectious disease cycle. It is defined as the period during which the symptoms felt by an infected individual may not be very specific or severe. The infected person can still perform usual functions and can therefore continue to be infectious. An infected individual can shed virus with very minor signs and symptoms.2 This explains why we are seeing widespread transmission in the community (this didn’t happen with SARS).
The reproductive number (R0)– the number of secondary infections generated from one infected individual – is estimated to be between 2 and 2.5 for COVID-19 virus.3 This means that a single infected person infects 2.5 people, which is higher than for the influenza virus. Approximately 3 to 5 days after infection, a person starts shedding virus and can infect others. Therefore, within a month, a single case can lead to 244 new cases.
The CDC now recommends social distancing to help decrease transmission of COVID-19 within the community.4 Social distancing measures, such as cancelling public gatherings and avoiding crowds, can slow the spread of the virus and spread cases out over a longer period of time, which can help hospitals provide care while avoiding being overwhelmed by patients. Social distancing helps “flatten the curve” in the spread of an infectious disease. This is especially critical because hospitals and health systems are working at full capacity. Flattening the curve ensures that systems are functional and people who require care the most can get the attention they need. The CDC recommends a distance of 6 feet or 2 meters as the minimum distance between individuals. 5
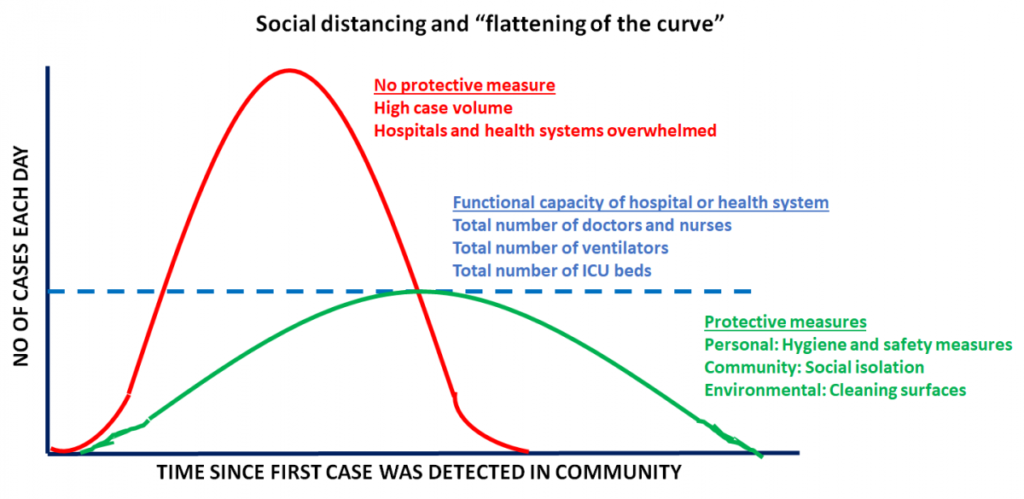
In case you are interested, we invite you to take a look at the CDC’s community transmission mitigation strategy document.
2. Appointments with your oncologist: virtual or in-person?
It may be a good idea to consider rescheduling or switching to a virtual appointment if your treating physician or cancer center provides this option. Note that virtual appointments are not appropriate for infusions for chemotherapy or immunotherapy, or potentially critical scans. If you have an oncology visit due in the next couple of months, please contact your treating physician as soon as possible to discuss what’s the right approach for you and whether they anticipate any drug shortages due to supply chain disruption.
Several hospitals are now limiting the number of visitors and/or people accompanying patients to no more than one at a time. In Seattle, the area hardest hit with cases, some hospitals are implementing the following measures to avoid being overwhelmed6:
- People with routine appointments are being screened for symptoms. Those who are sick are required to wear a mask or may not be allowed to enter the clinic.
- Elective surgeries are being postponed.
- Patients who have flu-like symptoms or other concerns are asked to CALL their doctor rather than going directly to the Emergency Department or Urgent Care.
- If you are having difficulty breathing, please do go to the Emergency Department.
3. COVID-19 testing: where we are now
We are seeing transmission in the community, so it’s likely the virus is more widespread in the United States than we imagine. However, we don’t have hard data because testing was not implemented in the earlier days of the epidemic in the US.7 Right now, most people need to have symptoms before they can be tested. As more test kits are distributed, testing will hopefully expand. Drive-through testing has been made available in a few locations but is not yet widely available. As we have stated previously, the symptoms of COVID-19 infection include fever, dry cough and shortness of breath. If you suspect that you have been infected, you should call your doctor or local health department to determine next steps. The availability of tests varies on where you live.
The CDC is maintaining an updated list of where tests are currently being performed in the US.8
Additionally, state health departments are a valuable resource, providing hotlines and websites with information about what to do if you are concerned that you or a loved one might be infected (links in the references).
4. Voices from the community
Please check out Janet Freeman-Daily’s article where she describes her experience as a lung cancer survivor with a cough and the difficulties she faced to get tested for COVID-19. Janet lives in the Seattle, WA area, a COVID-19 hotspot.
Fred Hutchinson Cancer Research Center in Seattle published a helpful blog post, “Coronavirus: what cancer patients need to know.”
References
- Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
- Hoehl S, Berger A, Kortenbusch M, et al. Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan, China. N Engl J Med. 2020.
- WHO. Coronavirus disease 2019 (COVID-19) Situation Report – 46. 2020.
- CDC. Implementation of Mitigation Strategies for Communities with Local COVID-19 Transmission Coronavirus Disease 2019 (COVID-19) Web site. cdc.gov/coronavirus/2019-ncov/downloads/community-mitigation-strategy.pdf. Published 2020. Accessed March 15, 2020.
- CDC. IInterim US Guidance for Risk Assessment and Public Health Management of Persons with Potential Coronavirus Disease 2019 (COVID-19) Exposures: Geographic Risk and Contacts of Laboratory-confirmed Cases. Coronavirus Disease 2019 (COVID-19) Web site. https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html. Published 2020. Accessed March 15, 2020.
- UW. COVID-19 (formerly called Novel Coronavirus). https://www.uwmedicine.org/coronavirus. Published 2020. Accessed 2020, March 15.
- Lambert J, Saey TH. Social distancing, not travel bans, is crucial to limiting coronavirus’ spread. Science News2020.
- CDC. Testing in U.S. Coronavirus Disease 2019 (COVID-19) Web site. https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html. Published 2020. Accessed March 15, 2020.
March 9, 2020 | Download the statement as a PDF
Update to the Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups | March 9
As advocacy organizations dedicated to serving the needs of lung cancer patients, all of us are closely monitoring the latest developments related to the outbreak caused by the novel coronavirus, SARS-CoV-2, and the resulting disease, COVID-19.
In this update, we have included additional information on facts about COVID-19, symptoms, testing, information about populations at risk of serious infection, and what you should do to protect yourself from COVID-19.
We are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC), which can be found here:
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- https://www.cdc.gov/coronavirus/2019-ncov/index.html
Facts about SARS-CoV-2/COVID-19
- This novel virus presents a unique threat to vulnerable populations, including the elderly and those with weakened immune systems, including cancer patients. Early studies conducted on lung cancer patients undergoing surgery suggest that this virus readily infects the lungs and can potentially cause pneumonia, making lung cancer patients particularly susceptible.1
- Research suggests that the overall clinical consequences of COVID-19 may ultimately be similar to those of a severe seasonal influenza or a pandemic influenza.2
- SARS-CoV-2 is very infectious. Infected individuals may not show symptoms of COVID-19 but are still considered infectious.3
Symptoms of COVID-19
Symptoms may appear 2-14 days after exposure and may include:
- Fever
- Tiredness
- Dry cough
- Some patients may have aches and pains, nasal congestion, runny nose, sore throat or diarrhea. These symptoms are usually mild and begin gradually.
- Some people become infected but don’t develop any symptoms and don’t feel unwell.
Can I get tested for COVID-19?
As of today, the CDC recommends testing symptomatic individuals. Clinicians should use their judgment to determine if a patient has signs and symptoms compatible with COVID-19 and whether the patient should be tested.4
Who are at increased risk of developing a serious form of COVID-19?
- Recent data suggest that certain populations may be at higher risk of getting very sick from infection with SARS-CoV-2. These groups include:
- People with cancer.5 Though the numbers are very small (5 out of 18 patients included in the study), research suggests that lung cancer patients may be susceptible to a more serious form of the infection
- People with lung disease,6 such as COPD (chronic obstructive pulmonary disease)
- People with hypertension (high blood pressure)7
- People with diabetes7
- People with heart disease6
- People with kidney disease8
- Older adults, defined as those above the age of 606,8
- People in active cancer treatment or whose immune systems may be compromised by chemotherapy or steroids9
- If you have more than one of the risk factors described above, you may be at an even greater risk of developing a serious form of COVID-19.
What you can do:
- If you or a loved one are in one of the high-risk groups described above:
- Stay at home as much as possible.
- Make sure you have access to several weeks of medications and supplies in case you need to stay home for prolonged periods of time.
- When you go out in public, keep away from others who are sick, limit close contact and wash your hands often.
- Avoid crowds, especially in poorly ventilated spaces.
- Stay up to date on CDC Travel Health Notices (https://wwwnc.cdc.gov/travel/notices).
- Plan now for what you will do if you, or people you rely on for support, become ill
- We encourage everyone to follow best practices for public health, such as staying home when ill, handwashing with soap and water (or using a hand sanitizer), and respiratory etiquette including covering the mouth and nose during sneezing and coughing.10 Many of the steps you would take to protect yourself from catching the flu also apply for protecting yourself against COVID-19.
- Regarding travel within the United States, at this time the CDC is encouraging limited travel restricted only to essential travel. We encourage all people to evaluate the need for non-essential travel and to take appropriate precautions if travel is required. Please check with your doctor before making international travel plans. Again, the CDC is maintaining a page that outlines current travel advisories: https://www.cdc.gov/coronavirus/2019-ncov/travelers/index.html
What you should not do:
Do not read or share information about COVID-19 from websites that are not maintained by reputable public health organizations (for example, the CDC). When in doubt, check your facts with what’s posted on the CDC or WHO website.
Treatment and Vaccines:
- As of now, there are no treatments for COVID-19. All treatments involve simply reducing the symptoms of the infection.
- As of now, there are no vaccines to prevent a COVID-19 infection.
References:
- Tian S, Hu W, Niu L, Liu H, Xu H, S. X. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. Journal of Thoracic Oncology. 2020.
- Fauci AS, Lane HC, Redfield RR. Covid-19 – Navigating the Uncharted. N Engl J Med. 2020.
- Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020.
- CDC. Evaluating and Reporting Persons Under Investigation (PUI) Summary of Recent Changes. Coronavirus Disease 2019 (COVID-19) Web site. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Published 2020. Accessed March 8, 2020.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337.
- CDC. People at Risk for Serious Illness from COVID-19. Coronavirus Disease 2019 (COVID-19) Web site. Published 2020. Accessed March 8, 2020.
- Guan W, Lian W, COVID-19 on behalf of China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1,590 patients with COVID-19 in China: A Nationwide Analysis. medRxiv. 2020.
- Liu Y, Sun W, Li J, et al. Clinical features and progression of acute respiratory distress syndrome in 2 coronavirus disease 2019 medRxiv. 2020.
- Center FHCR. Coronavirus: what cancer patients need to know. https://www.fredhutch.org/en/news/center-news/2020/03/coronavirus-what-cancer-patients-need-to-know.html. Published 2020. Accessed March 8, 2020.
- Del Rio C, Malani PN. 2019 Novel Coronavirus-Important Information for Clinicians. JAMA. 2020.
March 3, 2020 | Download the statement as a PDF
Joint Statement on Coronavirus COVID-19 from Lung Cancer Advocacy Groups
We understand and appreciate the severity of the new coronavirus epidemic (also known as COVID-19) that’s spreading globally. As advocacy organizations dedicated to serving the needs of lung cancer patients, all of us are closely monitoring the latest developments related to the outbreak caused by the novel coronavirus, SARS-CoV-2, and the resulting disease, COVID-19.
This is a rapidly evolving situation and we are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC), which can be found here.
Facts about SARS-Cov-2/COVID-19
- This novel virus presents a unique threat to vulnerable populations, including the elderly and those with weakened immune systems, including cancer patients. Early studies conducted on lung cancer patients undergoing surgery suggest that this virus readily infects the lungs and can potentially cause pneumonia, making lung cancer patients particularly susceptible.1
- Research suggests that the overall clinical consequences of COVID-19 may ultimately be similar to those of a severe seasonal influenza or a pandemic influenza.2
What you can do:
- First and foremost, we encourage everyone to follow best practices for public health, such as staying home when ill, handwashing with soap and water (or using a hand sanitizer), and respiratory etiquette including covering the mouth and nose during sneezing and coughing.3 Many of the steps you would take to protect yourself from catching the flu also apply for protecting yourself against COVID-19.
- Regarding travel within the United States, at this time there are no restrictions on travel. However, the situation may change rapidly. We encourage all people to evaluate the need for non-essential travel and to take appropriate precautions if travel is required. Please check with your doctor before making international travel plans. Again, the CDC is maintaining a page that outlines current travel advisories.
What you should not do:
- Do not read or share information about COVID-19 from websites that are not maintained by reputed public health organizations (for example, the CDC). When in doubt, check your facts with what’s posted on the CDC or WHO website.
References:
1. Tian S, Hu W, Niu L, Liu H, Xu H, S. X. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. Journal of Thoracic Oncology. 2020.
2. Fauci AS, Lane HC, Redfield RR. Covid-19 – Navigating the Uncharted. N Engl J Med. 2020.
3. Del Rio C, Malani PN. 2019 Novel Coronavirus-Important Information for Clinicians. JAMA. 2020.