There are two main types of lung cancer: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). The most common subtypes of NSCLC are adenocarcinoma (A-deh-noh-KAR-sih-NOH-muh) and squamous cell carcinoma (SKWAY-mus sel KAR-sih-NOH-muh). Beyond this general classification, your health care team may use biomarker testing to determine the best personalized treatment for you.

The information on this page is also available as a printed brochure. Click here to download or order.
In addition, a one-sheet quick guide to biomarkers is also available. The guide in web format can be found here.
Resources in Spanish are available at this link.
What is biomarker testing?
The NIf you have NSCLC, you and your doctor should discuss if biomarker testing is right for you. Biomarker testing (also called molecular testing, genomic testing, or molecular profiling) looks for mutations and other changes in the DNA of the tumor cells, as well as any changes in RNA or levels of specific proteins. RNA testing shows which genes are active in the tumor cells and may reveal changes that can’t be found by looking at DNA alone. Measuring protein levels in the tumor can help show whether certain proteins are overproduced. DNA testing is most common, but looking at RNA and protein levels together gives a more complete picture of what’s happening in the tumor.
Your cancer has DNA that is different from the DNA in your healthy cells. Biomarker testing looks for acquired mutations (DNA changes that happen during your lifetime) in your tumor—not inherited or germline mutations (changes that are passed to you from your parents).
If your doctor looks for all possible DNA mutations alone or along with RNA and protein levels, it’s called comprehensive biomarker testing. This approach is usually necessary for your doctor to provide treatment recommendations..
We all have DNA. Even your tumor.
What is a biomarker?
The National Cancer Institute defines a biomarker as a biological molecule found in blood, body fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease. With cancer, biomarkers are usually proteins, genes, or other molecules that influence how cancer cells grow, multiply, die, and respond to substances in the body. Cancer biomarkers can provide important clues about the cancer’s behavior and how it may respond to treatment.
In lung cancer, there are two main types of biomarkers that may help your doctor determine the best treatment plan for you:
- Oncogenic driver mutations
- Immunotherapy biomarkers
What are oncogenic driver mutations?
Oncogenic driver mutations are changes in the DNA sequence of cancer cells that help the cancer grow. Some of these mutations may cause the cancer to grow and divide uncontrollably.
Understanding DNA, RNA, and proteins
DNA (deoxyribonucleic acid) is like a blueprint, giving instructions to make the proteins that cells need to function. RNA (ribonucleic acid) acts like an architect, interpreting the DNA blueprint and directing the cells to build proteins.
DNA is not just a random jumble—it’s organized into genes, which are found on specific chromosomes inside the nucleus of each cell. Chromosomes contain all the instructions our bodies need to work and grow. Think of chromosomes like books in a library, where each book (chromosome) provides a set of instructions (genes) for making specific proteins that do different jobs in the body.
If a protein is abnormal, it could be due to a change, or mutation, in the DNA or because too much protein is made (overproduced).
There are different types of oncogenic driver mutations, including:
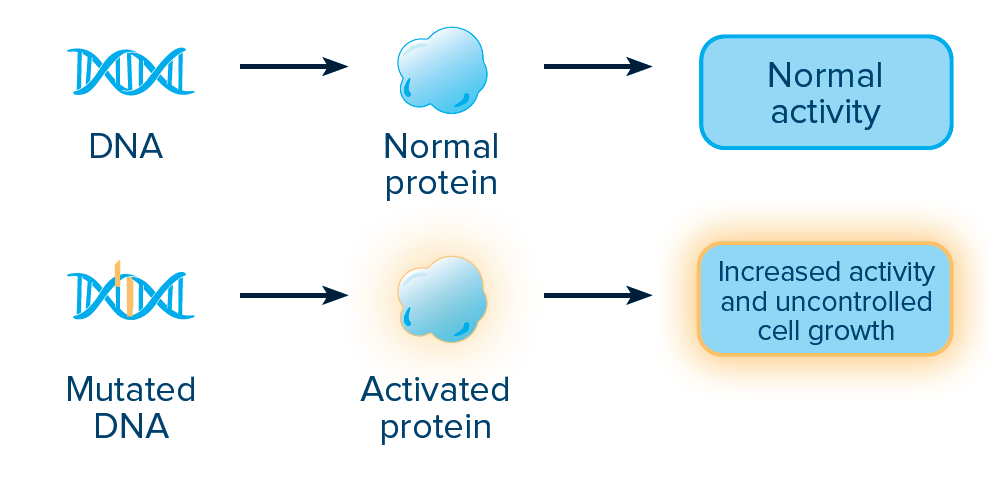
ACTIVATING MUTATIONS:
An activating mutation changes the DNA sequence in a way that makes the associated protein more active than normal. The DNA sequence can be changed by a point mutation, which happens when one nucleotide base is added (insertion), deleted (deletion), or changed (substitution).
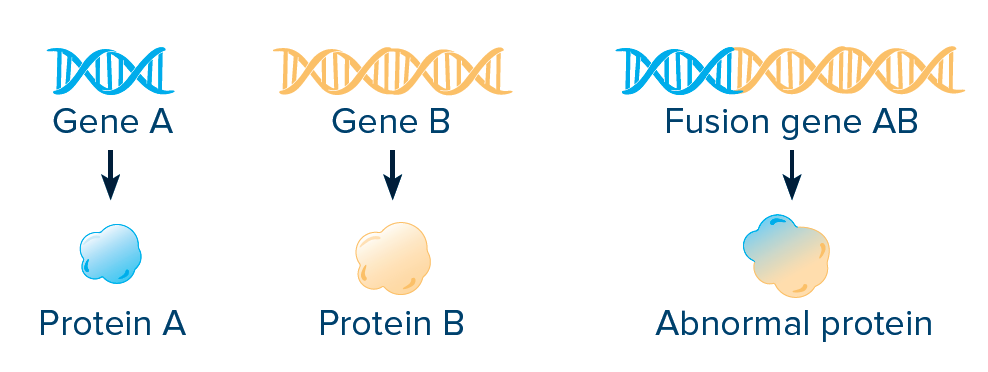
FUSIONS AND REARRANGEMENTS:
A fusion, or rearrangement, happens when a part of one gene fuses with (attaches to) part of another gene. The fusion gene then makes an abnormal protein, resulting in increased activity and increased growth of cancer cells.
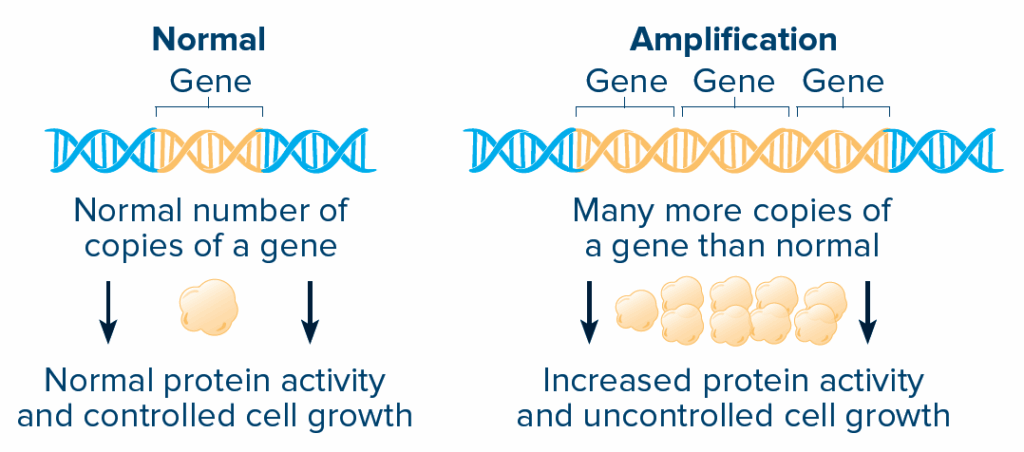
AMPLIFICATION AND OVEREXPRESSION:
Amplification means that there are many more copies of a gene than normal. The protein made from that gene is then overexpressed and can become more active.
What are immunotherapy biomarkers?
Immunotherapy biomarkers are used to determine whether you may benefit from a type of treatment called immunotherapy that works by boosting your body’s immune system to recognize and attack cancer cells. Immunotherapy biomarkers are often proteins or other molecules on the surface of cancer cells or immune cells. If these biomarkers are present, it can indicate that the cancer may respond well to immunotherapy.
PD-L1 is an immunotherapy biomarker that doctors commonly look for in patients with lung cancer. PD-L1 is a protein on tumor cells that interacts with the PD-1 receptor on immune system cells (T cells).
If you have high levels of PD-L1, your doctor may feel that a type of immunotherapy called an immune checkpoint inhibitor is the best treatment approach. Immune checkpoint inhibitors block, or inhibit, the interaction between PD-1 and PD-L1 to allow your immune system to recognize and attack cancer cells. Many patients who have oncogenic driver mutations may not respond well to immunotherapy.

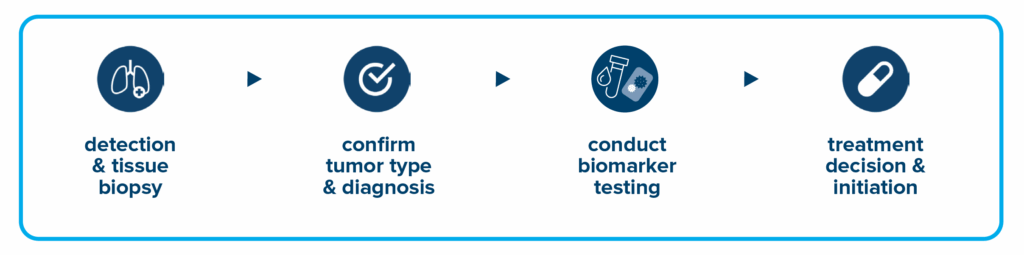
How is biomarker testing performed?
When your doctor suspects you have cancer, they take a small portion of your tumor tissue during a procedure called a biopsy to have it examined. A specialist, called a pathologist, looks at the tumor cells under a microscope to confirm a cancer diagnosis and performs additional tests that can tell where the tumor came from. The sample is then sent to a lab to be tested for biomarkers.
A liquid biopsy can be done on a sample of blood to look for cancer cells or for pieces of DNA from tumor cells that are circulating in the blood. A liquid biopsy may be used to help plan treatment, find out how well treatment is working, or determine if cancer has come back. However, a liquid biopsy is not always a substitute for a tissue biopsy and may miss mutations and other DNA changes that would have been detected in tissue-based tests, and it also requires advanced technology that may not be widely available.
How can biomarker information help plan my treatment?
It’s all about personalized medicine when it comes to lung cancer treatment. If a particular result is found through comprehensive biomarker testing, specific treatment options may be considered for you. The biomarkers may be actionable or nonactionable:
- Actionable biomarkers are biomarkers for which there is one or more approved drugs (targeted therapy). They may also be called actionable mutations.
- Nonactionable biomarkers do not have currently approved therapies, but there may still be benefits to finding them, such as the opportunity to participate in ongoing or future clinical trials specific to those targets.
What is targeted therapy?
Targeted therapies are drugs that target and block mutations to stop cancer growth. Imagine the drug works like a key fitting into a lock—it targets the mutation, blocking the cancer’s ability to grow. For example, if a tumor has a gene mutation causing rapid cell growth, targeted therapy may work to stop or slow down that growth.
What are the most common mutations?
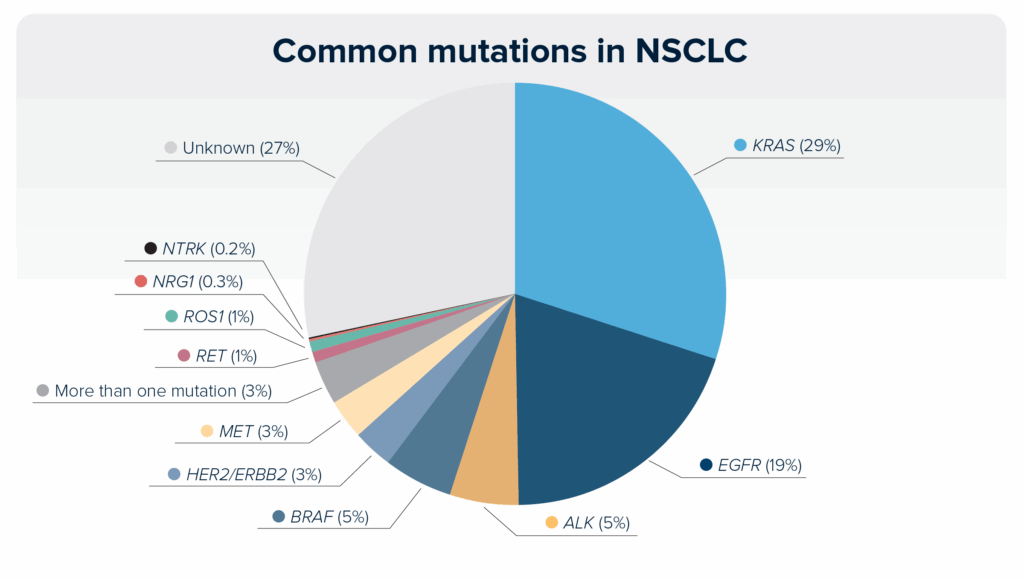
Some mutations are more common than others, and the incidence may differ based on the NSCLC subtype (adenocarcinoma vs squamous cell carcinoma). Although a mutation may be common, that does not mean it is actionable. Currently, the most common mutations are KRAS- and EGFR-activating mutations. This pie chart shows the incidence of common mutations in NSCLC. With the discovery of new mutations or new targeted therapies, this chart may change in the future.
Understanding KRAS and EGFR mutations
What is KRAS?
KRAS is a gene that provides instructions for making a protein also called KRAS. This protein plays an important role in controlling cell growth by signaling when cells should grow and when they should stop. KRAS is classified as an oncogene, meaning that it can cause cancer when it changes or mutates.
What are KRAS mutations?
When the KRAS gene is mutated, the KRAS protein is active, or “on,” all the time, leading to uncontrolled cell growth that can cause cancer. There are many types of KRAS mutations, each behaving differently, but the most common in lung cancer is the KRAS G12C mutation, which happens in about half of patients with lung cancer with a KRAS mutation.
How do KRAS mutations affect treatment?
Targeted treatments are linked to specific types of KRAS mutations. Currently, the only actionable KRAS mutation is KRAS G12C. Medicines called KRAS G12C inhibitors, taken by mouth, are designed to target and block the effects of the KRAS G12C mutation. These medicines work by sticking to the mutated KRAS protein, stopping it from causing uncontrolled cell growth. Research is ongoing to develop new treatments for other KRAS mutations.
What is EGFR?
EGFR is a gene that provides instructions for making EGFR (epidermal growth factor receptor), a protein that helps cells grow. When EGFR mutates, it can cause cells to grow uncontrollably, leading to cancer.
What are EGFR mutations?
Several types of EGFR mutations are linked to lung cancer. Some mutations are more common than others.
- Exon 19 deletions and exon 21 L858R mutations make up 80% to 90% of all EGFR mutations in NSCLC. These mutations happen in exons, which are segments of genes that contain protein-coding information. An exon 19 deletion happens when part of exon 19 is missing, which changes how the gene works. In an exon 21 L858R mutation, a single nucleotide in the DNA sequence of exon 21 is changed (substitution), changing the protein’s function and causing uncontrolled cell growth.
- An exon 20 insertion refers to when extra genetic material is inserted into exon 20 of the EGFR gene. Although rare (2% to 3% of all NSCLC cases), it is the third most common EGFR mutation in NSCLC.
- Other mutations include G719X in exon 18, L861Q in exon 21, and S768I in exon 20. These mutations happen less frequently but can still affect treatment options.
Knowing if your tumor has a unique trait, such as a targetable mutation, is important in helping you and your doctor make well‑informed decisions about your treatment.
Can a tumor have more than one mutation or more than one cancer subtype?
Cancers can have multiple mutations, known as co-mutations. In NSCLC, TP53 gene mutations are commonly found as co-mutations with EGFR mutations. Lung cancers with KRAS mutations may also have co-mutations in the TP53, KEAP1, and STK11 genes. Understanding co-mutations is important because they can affect how your cancer responds to treatment. There are some rare instances where a cancer has more than one subtype of NSCLC.
What kind of targeted therapies are available to treat NSCLC?
At the time of this publication, several targeted therapies have been approved to treat NSCLC, especially for common actionable mutations. Testing recommendations for actionable mutations may vary depending on availability, insurance coverage, and FDA-approval status.
| Gene | Oncogenic Mutation | Actionable: Targeted Therapy for Specific Mutations | FDA-Approved Drugs* |
|---|---|---|---|
| ALK | ALK rearrangements or fusions | ALK inhibitors target ALK gene rearrangements | Alectinib (ALECENSA®) Brigatinib (ALUNBRIG®) Ceritinib (ZYKADIA®) Crizotinib (XALKORI®) Ensartinib (ENSACOVE™) Lorlatinib (LORBRENA®) |
| BRAF | V600E activating mutation | BRAF/MEK inhibitors for V600E mutations | Dabrafenib + trametinib (TAFINLAR® + MEKINIST®) Encorafenib + binimetinib (BRAFTOVI® + MEKTOVI®) |
| BRAF | Non-V600E mutations | Drugs for non-V600E mutations are under evaluation in clinical trials | None |
| EGFR | Exon 19 and exon 21 mutations | EGFR inhibitors for mutations in exon 19 and exon 21 | Afatinib (GILOTRIF®) Amivantamab-vmjw + lazertinib (RYBREVANT® + LAZCLUZE™) Dacomitinib (VIZIMPRO®) Erlotinib (TARCEVA®) Gefitinib (IRESSA®) Osimertinib (TAGRISSO®) |
| EGFR | Exon 19 and exon 21 mutations | After EGFR inhibitors and chemotherapy | Datopotamab deruxtecan-dlnk (DATROWAY®) |
| EGFR | Exon 20 insertion mutations | EGFR inhibitors for exon 20 mutations | Amivantamab-vmjw + chemotherapy (RYBREVANT® + chemotherapy) Sunvozertinib (ZEGFROVY®) |
| EGFR | Other EGFR mutations | Discuss available treatment options with your oncologist | Afatinib (GILTOTRIF®) for certain uncommon EGFR mutations |
| HER2/ERBB2 | Exon 20 insertion mutations | HER2 targeted drug approved | Trastuzumab deruxtecan (ENHERTU®) |
| HER2/ERBB2 | Amplification | HER2 targeted drugs are under evaluation in clinical trials | None |
| HER2/ERBB2 | Overexpression | Drug approval for HER2 3+ overexpression | Trastuzumab deruxtecan (ENHERTU®) |
| HER2/ERBB2 | HER2/ERBB2 mutations | HER2 targeted drug approved | Zongertinib (HERNEXEOS®) |
| KRAS | G12C mutations | KRAS inhibitors for KRAS G12C mutation | Adagrasib (KRAZATI®) Sotorasib (LUMAKRAS®) |
| KRAS | Other KRAS mutations | Novel inhibitors for other KRAS mutations are in early phases of development | None |
| MET | Exon 14 skipping mutation | MET inhibitors for skipping mutations | Capmatinib (TABRECTA®) Tepotinib (TEPMETKO®) |
| MET | Overexpression | MET inhibitor for overexpression | Telisotuzumab vedotin (EMRELIS™) |
| MET | Amplification | MET inhibitors for cancers with MET amplification and overexpression are under evaluation | None |
| NRG1 | NRG1 fusions | NRG1 inhibitor | Zenocutuzumab (BIZENGRI®) |
| NTRK | NTRK1, NTRK2, NTRK3 fusions | Selective NTRK inhibitors | Entrectinib (ROZLYTREK®) Larotrectinib (VITRAKVI®) Repotrectinib (AUGTRYO™) |
| RET | RET rearrangement or fusions | RET inhibitors | Pralsetinib (GAVRETO®) Selpercatinib (RETEVMO®) |
| ROS1 | ROS1 fusions | ROS1 inhibitors for ROS1 fusions | Ceritinib (ZYKADIA®) Crizotinib (XALKORI®) Entrectinib (ROZLYTREK®) Lorlatinib (LORBRENA®)† Repotrectinib (AUGTYRO™) Taletrectinib (IBTROZI™) |
*This list reflects FDA approvals through September 2025 and is subject to change with continued research and trials. Drug names within each oncogenic mutation are listed in alphabetical order.
†Lorlatinib is not FDA-approved for ROS1-positive lung cancer but is recognized in certain clinical guidelines as a treatment option. Patients should discuss all available treatment options with their doctor.
Additional questions you may have
Will I need more than one biopsy?
Remember this—the tissue is usually the issue! If there is enough tissue from the original biopsy of your tumor, it can be tested. If not, you may need a second biopsy to get enough tissue to test. Your doctor may also recommend a liquid biopsy if there is not enough tissue, but it can sometimes miss tumor mutations. Your doctor may take multiple samples of blood over time to monitor what kind of molecular changes are taking place in your tumor.
A second biopsy may also be necessary if comprehensive biomarker testing wasn’t done initially, to examine disease progression, to track how your treatment is working, to test for any additional mutations, or to check if you are a candidate for any newly approved drugs or clinical trials.
Where can I go to get my tumor tested?
You don’t need to go to a specific place for testing; the tissue collected from your biopsy will be sent for testing for you. Many major medical centers offer biomarker testing in their own labs or have contracts with outside lab services. Private companies also perform this service.
What does tumor testing cost?
Most biomarker tests for lung cancer are covered by insurance. There are also financial assistance programs offered by labs that may help you afford testing. If you have concerns about whether your insurance will pay for biomarker testing, talk with your lung cancer care team for further guidance.
Eli Lilly & Company offers the Lilly Lung Cancer NGS Program, where eligible people can receive comprehensive biomarker testing at no cost. Download an information sheet in English or Spanish to learn more.
What if my test results don’t qualify me for targeted treatment?
Even if your tumor doesn’t have characteristics that can be matched to a targeted treatment, biomarker testing can still help your doctor to personalize your treatment. In these cases, chemotherapy suited for your type of cancer may be recommended, with or without immunotherapy.
Biomarkers for lung cancer are always under evaluation. Talk with your health care team about your biomarkers, your treatment options, and any ongoing clinical trials.
The information collected from biomarker testing may help determine if you can be considered for specific clinical trials. Clinical trials are devoted to learning about how biomarkers can guide treatment decisions and further the success of personalized medicine. Clinical trials for personalized medicine are designed to ensure that the right treatment can get to the right patient, at the right dose, at the right time.
Start the conversation
If you want to begin the discussion with your doctor, here are some questions to help guide the conversation:
- Do I need biomarker testing?
- What type of biomarker testing should I ask for?
- How could comprehensive biomarker testing affect my course of treatment?
- If I have a specific biomarker, does that mean I can only get treatment associated with that biomarker?
- Is there a clinical trial specific to my biomarker testing result?
- Is there enough biopsy tissue for this testing? Will I need another biopsy?
- How much does comprehensive biomarker testing cost? What is typically covered?
- How long will it take to get the results of this testing?
- Can I still get comprehensive biomarker testing even though I have already begun treatment?
Biomarker reports can be hard to interpret and understand, so be sure to discuss them with your health care team. If your doctor doesn’t recommend biomarker testing for you, it’s okay for you to ask, “why not?” There are cases where comprehensive biomarker testing will not be useful to your treatment plan. It’s best for you to know as much as you can about your disease so that you and your doctors can be full partners in your care. Remember that it’s always okay to ask for a second opinion from another doctor about anything related to your treatment and to learn more about clinical trials.

Biomarker Collaborative can help you locate specific biomarker support communities and resources: biomarkercollaborative.org
You can also find a list of patient groups at this link.
Have questions or need further information? Contact LCRF’s lung cancer support line at (844) 835-4325 or email support@LCRF.org.
LCRF provides these complimentary educational materials thanks to support from the following sponsors as well as generous donations:




Eli Lilly and Company
Foundation Medicine, Inc.
Gilead
Nuvation Bio
Summit Therapeutics, Inc.
TaihoOncology
Takeda Oncology