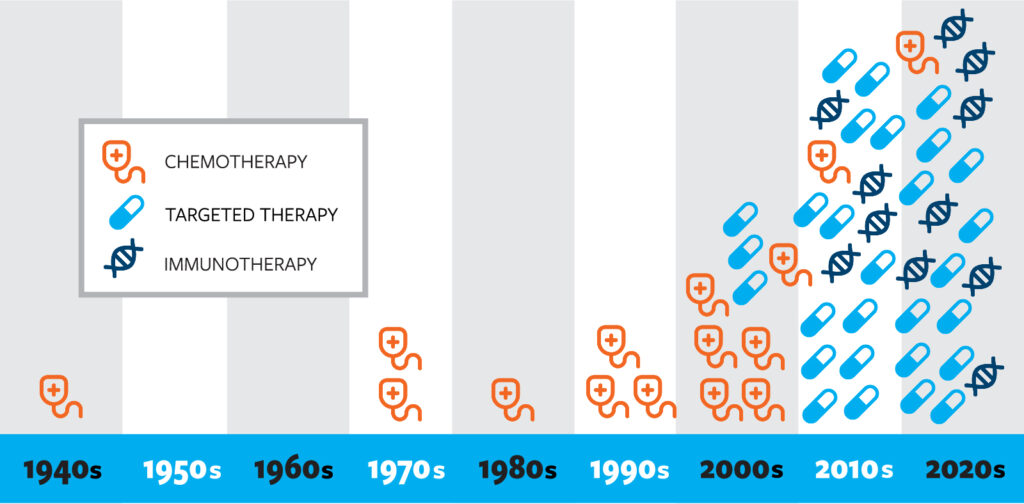
The following chart demonstrates how the pace of FDA approvals for lung cancer treatments has accelerated in recent decades. This rapid development is a result of scientific discoveries and continued progress made possible by funding research. See a list of drugs by decade
FDA approvals in the 2020s
Below is a list of recent FDA approvals for the treatment of lung cancer. In a relatively short time, we’re seeing an exponential increase in treatment approvals!
- NSCLC: non-small cell lung cancer
- SCLC: small cell lung cancer
- ALK: anaplastic lymphoma kinase
- EGFR: epidermal growth factor receptor
- MET: mesenchymal-epithelial transition
- RET: rearranged during transfection
2024
October
- FDA approved nivolumab (Opdivo®) with platinum-doublet chemotherapy as neoadjuvant treatment, followed by single-agent nivolumab after surgery as adjuvant treatment for NSCLC with no EGFR or ALK mutations. Link
September
- FDA approved osimertinib (Tagrisso®) for patients with stage 3 NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations. Link
- FDA approved amivantamab-vmjw (Rybrevant®) with carboplatin and pemetrexed for patients with EGFR exon 19 deletions or exon 21 L858R substitution mutations whose disease progressed on or after treatment with an EGFR tyrosine kinase inhibitor. Link | Science Made Simple
- FDA approved atezolizumab (Tecentriq®) and hyaluronidase-tqjs (Hybreza) for subcutaneous injection to treat NSCLC and SCLC. Link
August
- FDA approved lazertinib (Lazcluze®) in combination with amivantamab-vmjw (Rybrevant®) for first-line treatment of locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations. Link
May
- FDA granted accelerated approval to tarlatamab-dlle (Imdelltra®) for extensive stage SCLC with disease progression or after platinum-based chemotherapy. Link | Science Made Simple
April
- FDA approved alectinib (Alecensa®) for adjuvant treatment following tumor resection in patients with ALK+ NSCLC. Link | Science Made Simple
March
- FDA approved amivantamab-vmjw (Rybrevant®) with carboplatin and pemetrexed for the first-line treatment of locally advanced or metastatic NSCLC with EGFR exon 20 mutations. Link
February
- FDA approved osimertinib (Tagrisso®) with platinum-based chemotherapy for patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations. Link | Science Made Simple
- FDA approved tepotinib (Tepmetko) for patients with MET exon 14+ NSCLC. Link | Science Made Simple
2023
November
- FDA approved repotrectinib (Augtyro®) for locally advanced or metastatic ROS1+ NSCLC. Link | Science Made Simple
October
- FDA approved pembrolizumab (Keytruda®) with platinum-containing chemotherapy as neoadjuvant treatment, and with continuation of single-agent pembrolizumab as post-surgical adjuvant treatment for resectable NSCLC tumors. Link
- The FDA has approved encorafenib (Braftovi®) plus binimetinib (Mektovi®) for adult patients with metastatic NSCLC harboring a BRAF V600E mutation. Link
August
- FDA granted regular approval to pralsetinib (Gavreto®) for adult patients with metastatic rearranged during transfection (RET) fusion-positive NSCLC. Link
January
- FDA approved pembrolizumab (Keytruda®) for adjuvant treatment following resection and platinum-based chemotherapy for stage 1B (T2a ≥4 cm), 2, or 3A NSCLC. Link
2022
December
- FDA granted accelerated approval to adagrasib (Krazati®), a RAS GTPase family inhibitor, for adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC who have received at least one prior systemic therapy. Link | Science Made Simple
November
- FDA approved cemiplimab-rwlc (Libtayo®) in combination with platinum-based chemotherapy for adult patients with advanced NSCLC with no EGFR, ALK, or ROS1 aberrations. Link
- FDA approved tremelimumab (Imjudo®) in combination with durvalumab (Imfinzi®) and platinum-based chemotherapy for adult patients with metastatic NSCLC with no sensitizing EGFR mutation or ALK genomic tumor aberrations. Link | Science Made Simple
September
- FDA granted regular approval to selpercatinib (Retevmo®) for adult patients with locally advanced or metastatic NSCLC with a RET gene fusion. Link
August
- FDA granted regular approval to capmatinib (Tabrecta®) for adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have a mutation leading to MET exon 14 skipping. Link
- FDA granted accelerated approval to fam-trastuzumab deruxtecan-nxki (Enhertu®) for adult patients with unresectable or metastatic NSCLC whose tumors have activating human epidermal growth factor receptor 2 HER2 (ERBB2) mutations, and who have received a prior systemic therapy. This is the first drug approved for HER2-mutant NSCLC. Link | Science Made Simple
March
- FDA approved nivolumab (Opdivo®) with platinum-doublet chemotherapy for adult patients with resectable NSCLC in the neoadjuvant setting. Link
2021
October
- FDA approved atezolizumab (Tecentriq®) for adjuvant treatment following resection and platinum-based chemotherapy in patients with stage 2 to 3A NSCLC whose tumors have PD-L1 expression on ≥ 1% of tumor cells. Link
September
- FDA approved mobocertinib (Exkivity®) for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations whose disease has progressed on or after platinum-based chemotherapy. Link
May
- FDA approved amivantamab-vmjw (Rybrevant™) for adults with NSCLC with EGFR exon 20 insertion mutations. Link
- FDA approved sotorasib (Lumakras™) as the first treatment for NSCLC patients whose tumors have the KRAS G12C genetic mutation and who have received at least one prior systemic therapy. Link
March
- FDA approved lorlatinib (Lorbrena®) targeted therapy drug now for the first-line treatment of NSCLC patients with the ALK mutation. Link
February
- FDA approved cemiplimab-rwlc (Libtayo®) immunotherapy drug for first-line treatment for NSCLC patients with high PD-L1 expression and who are not eligible for surgery or chemoradiation. Link
- FDA approved trilaciclib (Cosela™), a drug that reduces chemotherapy-induced bone marrow suppression, for SCLC patients. Link
- FDA approved tepotinib (Tepmetko®) for the treatment of metastatic NSCLC patients who have the MET exon 14 skipping alterations. Link
2020
December
- FDA approved osimertinib (Tagrisso®) for adjuvant therapy for NSCLC patients who have undergone resection and have tumors positive for either EGFR exon 19 or exon 21 L858R. Link
October
- FDA approved FoundationOne CDx companion diagnostic test to help identify NTRK+ solid tumor patients eligible for larotrectinib and to help identify ALK+ patients eligible for alectinib. Link
- FDA approved “cobas EGFR mutation test v2” to identify NSCLC patients eligible for any of the EGFR inhibitor therapies, including those used to treat EGFR exon 19 and L858R deletions, as well as any EGFR therapies to come in the future. Link
September
- FDA approved pralsetinib (Gavreto®) for people with metastatic NSCLC who have the MET exon 14 mutation. Link
August
- FDA approved Guardant360 CDx assay, a liquid biopsy (blood test) that also uses next-generation sequencing (NGS) technology to identify patients with the EGFR mutation. Link
June
- FDA approved lurbinectedin (Zepzelca®) for the second-line treatment of patients with metastatic SCLC. Link
May
- FDA approved combination ramucirumab and erlotinib for first-line treatment of NSCLC patients with the EGFR exon 19 or exon 21 mutations. Link
- FDA approved combination nivolumab (Opdivo®) and ipilimumab (Yervoy®) for the first-line treatment of patients with PD-L1+ metastatic NSCLC. This combination can also be used as a first-line treatment in conjunction with chemotherapy for metastatic NSCLC. Link
- FDA approved brigatinib (Alunbrig®) as a first-line treatment for metastatic NSCLC patients with the ALK mutation. Link
- FDA approved atezolizumab (Tecentriq®) as a first-line treatment for metastatic NSCLC patients who have high PD-L1 expression. In conjunction with this approval, the FDA approved the diagnostic test Ventana PD-L1 assay. Link
- FDA approved capmatinib (Tabrecta®) as a treatment for metastatic NSCLC patients who have the MET exon 14 mutation. In conjunction with this approval, the FDA approved the diagnostic test FoundationOne CDx assay. Link
- FDA approved selpercatinib (Retevmo®) as a treatment for patients with NSCLC or thyroid cancer who have the RET mutation. Link
- FDA approved nivolumab (Opdivo®) plus ipilimumab (Yervoy®) and chemotherapy for first-line treatment of metastatic NSCLC. Link
March
- FDA approved durvalumab (Imfinzi®) in combination with chemotherapy as a first line treatment for extensive stage SCLC. Link
February
- FDA approved pemetrexed (Pemfexy®) injectable chemotherapy for treatment of patients with advanced non-squamous NSCLC. Link
Approved drugs by decade
1940s
Mechlorethamine Hydrochloride
1970s
Doxorubicin Hydrochloride
Methotrexate Sodium
1980s
Cisplatin
1990s
Etoposide / Etoposide Phosphate
Gemcitabine Hydrochloride
Vinorelbine Tartrate
2000s
Bevacizumab
Carboplatin
Docetaxel
Erlotinib Hydrochloride
Everolimus
Gefitinib
nab-Paclitaxel
Pemetrexed Disodium
Topotecan Hydrochloride
2010s
Afatinib Dimaleate / Afatinib
Alectinib
Atezolizumab
Brigatinib
Ceritinib
Crizotinib
Dabrafenib Mesylate
Dacomitinib
Durvalumab
Entrectinib
Larotrectinib
Lorlatinib
Necitumumab
Nivolumab
Osimertinib Mesylate
Pembrolizumab
Ramucirumab
Trametinib Dimethyl Sulfoxide
2020s
Adagrasib
Amivantamab-vmjw
Binimetinib
Capmatinib Hydrochloride
Cemiplimab-rwlc
Encorafenib
Fam-trastuzumab deruxtecan-nxki
Ipilimumab
Lurbinectedin
Mobocertinib Succinate
Pralsetinib
Selpercatinib
Sotorasib
Tepotinib Hydrochloride
Tremelimumab
Trilaciclib