LCRF publishes a quarterly e-newsletter highlighting the latest developments in the lung cancer space and announcing upcoming events. The e-news also features stories from patients and supporters.
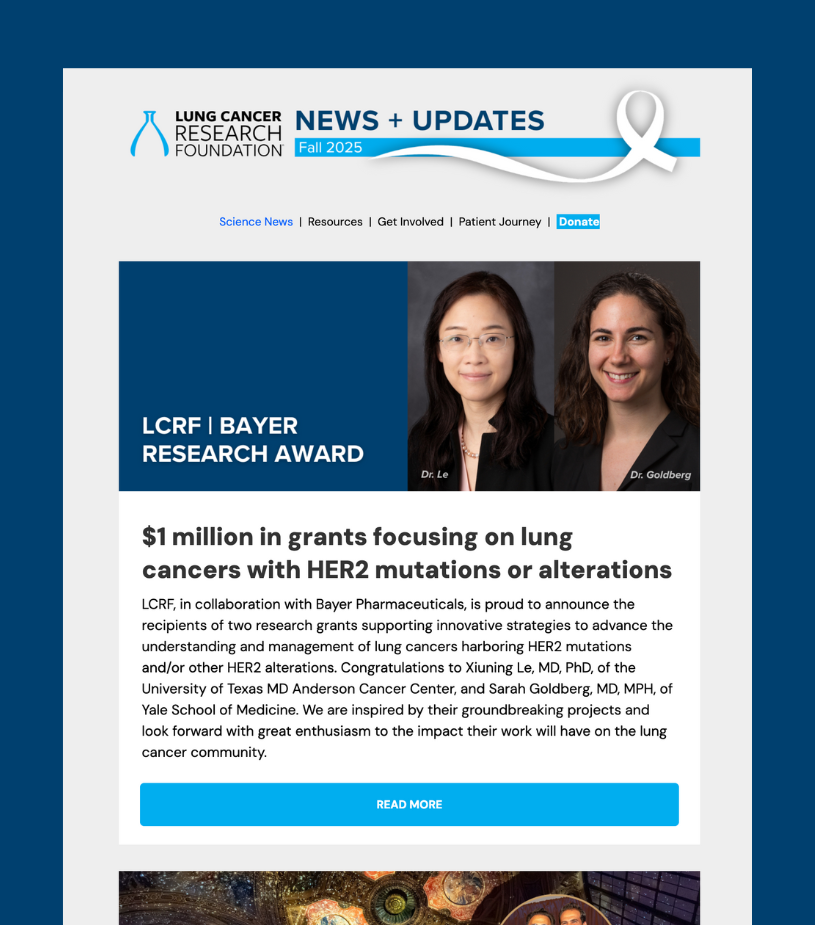
NEW YORK, NY (September 30, 2025) – The Lung Cancer Research Foundation (LCRF) is pleased to announce, in collaboration with Bayer Pharmaceuticals, the recipients of two research grants focused on innovative strategies to advance the understanding and management of lung cancers harboring HER2 mutations and/or other HER2 alterations.
These grants are awarded to projects that address important mechanistic questions and developmental therapeutics across the care continuum for HER2-mutant NSCLC, with the potential to improve patient outcomes. Titled LCRF | Bayer Research Award on Innovative Therapeutic Strategies to Treat Lung Cancers Harboring HER2 Mutations and/or Other HER2 Alterations, submissions were required to include correlative, translational research to advance the understanding of HER2-driven lung cancers. Additionally, submissions were required to include a patient or patient advocate as part of the research team with a role in project design. Awardees receive $500,000 over two years for their projects.
“There is an urgent need to understand the role of HER2 alterations as oncogenic drivers as well as tumor response and resistance mechanisms,” says Kathryn O’Donnell, PhD, LCRF Scientific Advisory Board chair. “We are hopeful that these projects will drive tangible benefits for patients whose tumors harbor these mutations or alterations.”
“We extend our congratulations to the recipients of these awards. We share the investigators’ commitment to patients living with lung cancer, and the desire to bring impactful research results to not only improve treatments, but quality of life,” said Lucia Regales, PhD, Global Medical & Evidence Strategy Lead Oncology at Bayer’s Pharmaceuticals Division.
The recipients of the LCRF | Bayer Research Award on Innovative Therapeutic Strategies to Treat Lung Cancers Harboring HER2 Mutations and/or Other HER2 Alterations are:

Sarah Goldberg, MD, MPH
Yale School of Medicine
Associate Professor of Internal Medicine (Medical Oncology)
Associate Director, Hematology/Oncology Fellowship Program
Research Director, Center for Thoracic Cancers
Stratifying and Personalizing for HER2 Mutated Lung Cancers

Xiuning Le, MD, PhD
University of Texas MD Anderson Cancer Center
Associate Professor, Department of Thoracic/Head and Neck Medical Oncology
Division of Internal Medicine
Characterization of HER2 Mutations’ Sensitivity to Sevabertinib & Development of Novel Combination Strategies to Overcome Resistance to Current HER2 Therapies
To learn more about LCRF funded research and its grants program, visit LCRF.org.
# # #
About the Lung Cancer Research Foundation (LCRF)
The Lung Cancer Research Foundation® (LCRF) is the leading nonprofit organization focused on funding innovative, high-reward research with the potential to extend survival and improve quality of life for people with lung cancer. LCRF’s mission is to improve lung cancer outcomes by funding research for the prevention, diagnosis, treatment, and cure of lung cancer. To date, LCRF has funded 431 research grants, totaling nearly $49 million, the highest amount provided by a nonprofit organization dedicated to funding lung cancer research. For more information, visit LCRF.org.
Contact:
Sheila Sullivan
Sr. Director, Marketing & Communications, LCRF
ssullivan@LCRF.org
To learn more about HER2-mutant NSCLC, download this educational resource. If you have questions, you can connect with our Lung Cancer Support Line at (844) 835-4325 or email support@LCRF.org.
More than 300 people gathered at Longview Lake in Kansas City on September 27 to walk toward a world without lung cancer.
Together, walkers raised over $35,000 to fund lung cancer research. Many were there to support family or friends experiencing lung cancer. Some had received a diagnosis themselves. Others were there to honor the memory of someone lost to the disease. Everyone held in common a commitment to research that will lead to better outcomes for patients and families.

Kudos to our top fundraising teams: The Lung and the Restless, Team Alexis Mirakian, and #TeamShelton. A high five to our top individual fundraisers as well: Alexis Mirakian, Wynter Heinen, and Stephanie Brunkhorst.
The walk couldn’t have happened without our Kansas City volunteer committee – Stephanie Brunkhorst, Steve Shelton, Jennifer Crosby, and Shana Abdullah – and our sponsors:
Our in-kind sponsors included Party It Up Entertainment along with FIT Muscle & Joint Clinic.
There’s still time to get involved! The Free to Breathe Anywhere Walk participants can walk any time they’d like through the end of 2025.
Glenna is a wife, mother, grandmother, and avid traveler… and she is living with lung cancer.
Her story started in December 2022, when she developed a nagging cough. She wasn’t especially worried, since she had been given a diagnosis of silent reflex some 8 years earlier. By March, however, the cough was getting worse.
She met with her primary care provider, who adjusted her medication. But 4 weeks later, her symptoms hadn’t improved. This time, she was referred to a specialist for reflux.
Then Glenna and her husband took an Alaskan cruise, and Glenna became very sick. Upon returning in June, she felt terrible and had severe pain in her shoulder blade. Nothing abnormal was found, but at that point Glenna hadn’t had a chest X-ray.
In late June, Glenna had quite a scare. “We were at home, and I told my husband he needed to take me to the ER immediately, because I couldn’t breathe.” The ER visit revealed a 9 cm mass in her lung, and she was admitted to the hospital for four days.
Glenna finally had a diagnosis: stage 4 non-small cell lung cancer. Her first-line chemotherapy treatment unfortunately failed. But a blood and tissue biopsy for biomarker testing after her initial diagnosis confirmed that her cancer had the HER2 mutation.
In September 2023, she began a targeted treatment, Enhertu. Glenna has continued on this treatment, and her status is officially NED – no evidence of disease.

Glenna and her husband have continued to travel throughout her diagnosis. “We had a lot of trips this summer, and we’re still planning more,” she said. “My family has been a great source of support through my treatment.”
“I’m glad I had testing done at the beginning, because it helped avoid a delay in care after my first-line treatment failed. I was relieved to have the additional information, as I knew it would give me options for targeted treatment for the HER2 mutation.”
> Learn about biomarker testing for HER2 and other genetic mutations
> Find a patient support group
> Donate to fund lung cancer research
Stephen Liu, MD; Rachel Sanborn, MD; and our moderator, Isabel Preeshagul, DO, MBS; discussed developments in lung cancer research and treatment that were presented at the 2025 World Conference on Lung Cancer.
Watch the recording below.
NEW YORK, NY (September 23, 2025) – The Lung Cancer Research Foundation (LCRF) and Oncology Advocates United for Climate and Health – International (OUCH-I) are partnering to fund projects that examine the impact of environmental pollution and climate change on lung cancer risk, diagnosis, treatment and outcomes; along with innovative strategies to mitigate these effects. The program, titled OUCH-International and LCRF Research Grant Program on the Effects of Air Pollution and Climate Change on Carcinogenesis and Lung Cancer Prevalence, is sponsored by AstraZeneca. A $200,000 award over a two-year period will be granted, and the Request for Proposals will open early in 2026.
Increasing evidence indicates that air pollution is a major cause of lung cancer, and the number of estimated lung cancer deaths attributable to air pollution has increased by nearly 30% since 2007, as smoking has decreased and air pollution has increased (Turner MC et al, CA Cancer J Clin, 2020). The International Agency for Research on Cancer (IARC) has classified outdoor air pollution and particulate matter (PM) with a diameter of less than 2.5 microns (1 inch = 25,400 microns) as a cause of lung cancer (Straif K et al, IARC Press, 2013; Loomis D et al, Lancet Oncol, 2013; GBD 2019 Risk Factors Collaborators, Lancet, 2020). Data now show that exposure to pollution—whether from industrial sources or wildfires—increases the risk of lung cancer in both smokers and non-smokers (Hill et al, Nature, 2023). Globally, outdoor (ambient) air pollution is regarded as the second most important cause of lung cancer mortality, and indoor air pollution is considered the seventh most important cause (GBD 2019 Risk Factors Collaborators, Lancet, 2020).
Air pollution causes many health hazards, can contribute to the development of lung cancer, and can worsen its prognosis. Awareness that exposure to air pollution is the second-largest risk factor for lung cancer needs to be at the forefront of the lung cancer community—to recognize its risks and to educate patients and the healthcare community at large.
“Understanding and identifying ways to mitigate the risk of developing lung cancer as a result of air pollution is a pressing issue,” remarked Joan Schiller, MD, Founder of OUCH-International and long-time member of LCRF’s Board of Directors. “Bringing together LCRF and OUCH-International to award these grants is an important step in increasing early detection of lung cancer. I’m grateful to AstraZeneca for sponsoring this very important program.”
“AstraZeneca is proud to partner with LCRF and OUCH-International in such a crucial area of lung cancer research,” said Nabil Chehab, Medical Affairs Head of AstraZeneca’s Lung Cancer Franchise. “Unlocking the impacts of pollution and climate change on lung cancer will enable the oncology community to better support education, detection, diagnosis, and treatments for every patient at every stage. This partnership will undoubtedly enhance our understanding and enable another step forward toward increasing survival for people living with lung cancer.”
“This collaboration will help us to understand how the air we breathe can increase our risk for developing lung cancer,” said Aubrey Rhodes, Executive Director of LCRF. “Dr. Schiller and her OUCH-International colleagues’ expertise and pursuit of solutions for the lung cancer community will help to improve the body of research that informs how lung cancer is detected, diagnosed, and treated.”
# # #
About the Lung Cancer Research Foundation (LCRF)
The Lung Cancer Research Foundation® (LCRF) is the leading nonprofit organization focused on funding innovative, high-reward research with the potential to extend survival and improve quality of life for people with lung cancer. LCRF’s mission is to improve lung cancer outcomes by funding research for the prevention, diagnosis, treatment, and cure of lung cancer. To date, LCRF has funded 431 research grants, totaling nearly $49 million, the highest amount provided by a nonprofit organization dedicated to funding lung cancer research. For more information about the LCRF grant program and funding opportunities, visit LCRF.org/research.
About Oncology Advocates United for Climate and Health – International (OUCH-I)
OUCH is the only non-profit, nonpartisan volunteer cancer organization focused on mitigating the effects of climate change on cancer care. Our mission is to advance awareness, actions, and policies that mitigate the effects of climate change on cancer care, and our pillars include advocacy, education and outreach research, cancer care delivery, sustainability, and resilience, and climate justice and health equity. Our members include oncology health care professionals (e.g. MDs, PhDs, RNs, pharmacists, social workers, researchers, patient advocates) who are interested in mitigating the effects of climate change on cancer. We currently have over 150 members representing 27 states and 23 different countries, including six oncology professional societies.
Contact:
LUNG CANCER RESEARCH FOUNDATION
Sheila Sullivan
Sr. Director, Marketing & Communications
ssullivan@LCRF.org
OUCH – INTERNATIONAL
Joan H. Schiller, MD
608-469-6992
joanhschiller@gmail.com
ouchforclimate.org
The Lung Cancer Research Foundation’s annual Evening of Innovation gala celebrated scientific discovery and the remarkable people who have contributed to the progress being made in the lung cancer space. The event raised more than $1.6 million to accelerate research, improve outcomes, and bring hope to countless families affected by lung cancer.
This year’s gala, held at Cipriani 25 Broadway on Sept. 17, honored Raymond E. Chalmé, a dedicated advocate for lung cancer research. Ray, along with his family, founded Elliot’s Legacy – a community kite-flying event named for his father, who died of lung cancer at the age of 56. Elliot’s Legacy has raised more than $8.5 million over the past 18 years.
Joan H. Schiller, MD, received the Founders Award for her visionary work to improve lung cancer outcomes. Dr. Schiller gave a voice to the lung cancer community when she founded Free to Breathe, a nationwide movement raising awareness and funds to support critical research.


Benay Taub shared a powerful and poignant personal story of her experience receiving a lung cancer diagnosis just last year. Her words touched everyone in the room as she called for all the attendees to support lung cancer research funding.
Will Reeve, the son of Christopher and Dana Reeve, made a surprise appearance, sharing with attendees about his mother’s experience with her diagnosis of Stage 4 lung cancer as well as their experience as a family, both through the diagnosis and the loss of his mother from the disease.
While this year’s event was a celebration of the progress made in lung cancer research, each of the speakers underscored the urgent need for more funding for lung cancer – it remains the deadliest of all cancers and the most underfunded. While we have come so far, there is still much work to be done.
Watch a short video about our honoree:
Hear remarks from our honoree and speakers:
Click a photo below to enlarge









LCRF’s science team talks about the role of the Scientific Advisory Committee and the importance of involving young scientists in lung cancer research. Watch the video below.
Featured:
Aubrey Rhodes, LCRF Executive Director
Dhru Deb, PhD, Senior Director, Research & Administration
Antoinette (Toni) Wozniak, MD, Chief Scientific Officer
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to zongertinib for the treatment of adult patients with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) whose tumors have HER2 (ERBB2) mutations and who have received prior systemic therapy. This is the first oral tyrosine kinase inhibitor (TKI) that has been approved for this indication.
Alterations in the HER2 gene have been associated with the development and spread of cancer. HER2 mutations occur in about 2-4% of patients with NSCLC. In the past two decades, several clinical trials have investigated the use of anti-HER2 therapies in lung cancer but have led to disappointing results. In 2022, the FDA granted accelerated approval to trastuzumab deruxtecan for patients with unresectable or metastatic NSCLC whose tumors have HER2 mutations and who have received prior therapy. This drug is an antibody drug conjugate, which is a form of “targeted chemotherapy”, and is the only other agent approved for HER2 mutated NSCLC.
Zongertinib is an oral TKI that specifically targets HER2. In the Beamion LUNG-1 (NCT04886804) clinical trial 75 patients with previously treated, metastatic NSCLC containing a HER2 mutation were given zongertinib. An impressive 71% of the patients had significant shrinkage of their cancer, and the response lasted for over a year (14 months) for many of the patients. (NEJM, 2025) The most common side effects included diarrhea, liver toxicity, rash, fatigue, and nausea. Of note, pneumonitis (inflammation of the lungs) was not seen as a side effect.
Zongertinib represents a large step forward in the treatment of NSCLC patients with HER2 mutations. Since this is an accelerated FDA approval there will be more trials that will be performed to confirm the effectiveness of the drug.
It is likely that zongertinib will be evaluated as initial treatment for patients with HER2 mutated NSCLC in upcoming clinical trials with the hope that it will be more effective than chemotherapy as a first treatment. We could also see the evaluation of zongertinib in combination with other agents such as chemotherapy. It is also important to remember that the drug is unlikely to be a cure for these patients and that there is still an urgent need to continue research efforts to determine why cancer cells are or become resistant to treatment. Expect that the development of novel drugs for this type of lung cancer will continue in the future.

The good news
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to sunvozertinib for the treatment of adult patients with advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, whose disease has progressed on or after platinum-based chemotherapy.
Why it’s important
EGFR mutations are present in 15-20% of NSCLC. These mutations are drivers of cancer development and have been successfully targeted with tyrosine kinase inhibitors (TKIs) that reduce cancer growth and improve patient survival. Not all EGFR mutations are created equally. The most common EGFR mutations are in exons 19 and 21 and they are the most responsive to treatment with the EGFR-TKIs. EGFR exon 20 insertion mutations represent about 10% of all EGFR mutations. The agents available for the treatment of the more common EGFR mutant lung cancers have not been as successful in most of the lung cancers that harbor an EFGR mutation. The current first-line treatment of advanced EGFR exon 20 insertion mutant lung cancer is amivantamab (an antibody that binds to both EGFR and MET) combined with chemotherapy. There are no approved treatments after the initial therapy fails.
Sunvozertinib is an oral TKI that specifically targets the EGFR exon 20 insertion mutation. In the WU-KONG1B (NCT03974022) clinical trial 85 patients with previously treated, metastatic NSCLC containing EGFR exon 20 insertion mutations were given sunvozertinib. Nearly half of the patients (46%) had significant shrinkage of their cancer, and the response lasted for nearly a year for many of the patients. The side effects included gastrointestinal toxicities (diarrhea, constipation, nausea, vomiting), skin rash and dryness, mouth sores, eye problems, fatigue, and pneumonitis (inflammation of the lungs).
What it means for patients
NSCLC with EGFR exon 20 insertion mutations have been difficult to treat because they do not respond well to the EGFR TKIs used for lung cancers containing the more common EGFR mutations. The FDA approval of sunvozertinib represents some progress in the treatment of this subtype of NSCLC.
What to look for
There will likely be more trials evaluating sunvozertinib in combination with other agents or possibly as initial therapy for patients with advanced NSCLC who have EGFR exon 20 insertion mutations. Expect that the development of novel drugs for this particular type of lung cancer will continue.